Overview
Dosing for RYBREVANT® + chemotherapy
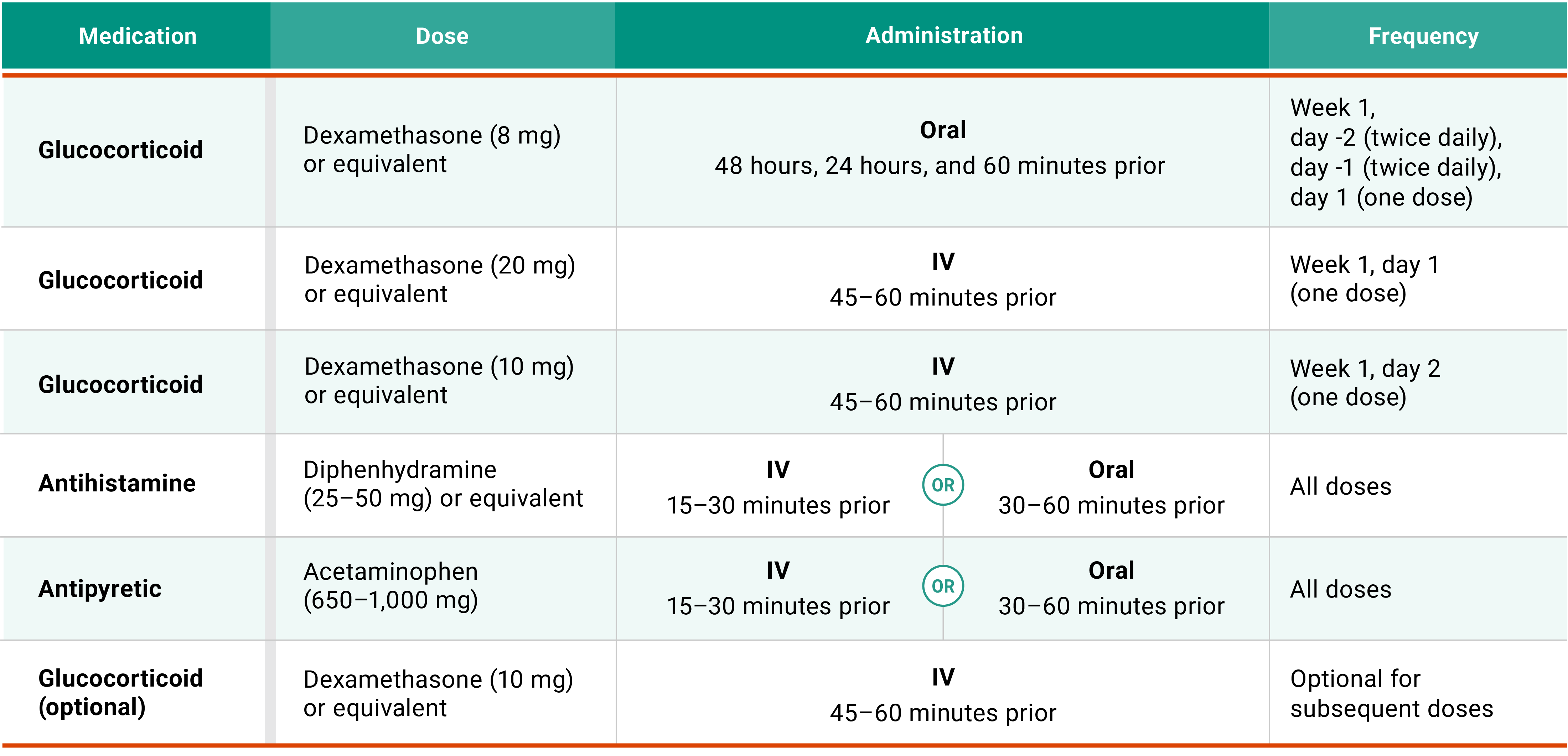
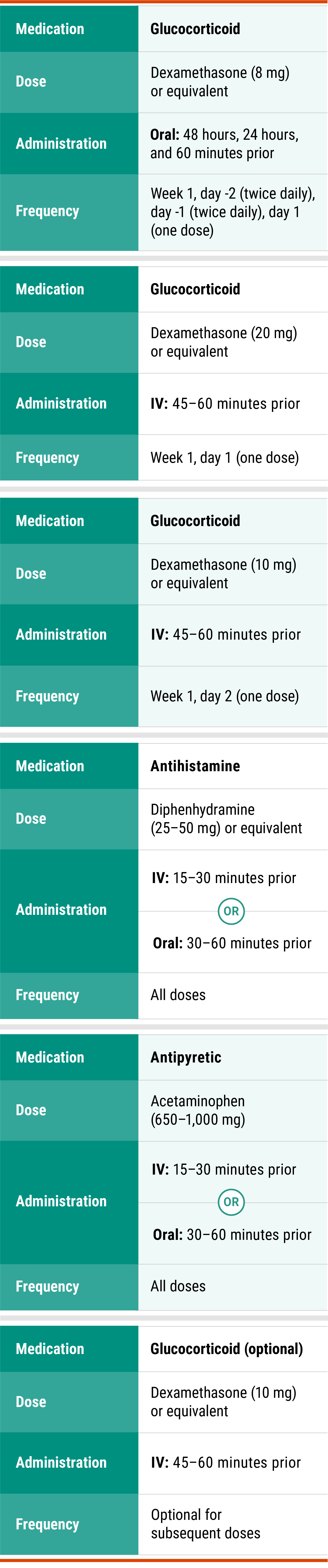
Premedications1
Administer antihistamine, antipyretic, and glucocorticoids*:
15 to 60 minutes prior to each infusion according to the recommended premedications in the table below.
*Glucocorticoid administration is required for week 1, days -2, -1, 1, and 2 doses only and upon reinitiation after prolonged dose interruptions, then as necessary for subsequent infusions.
See recommended premedicationsDecreasing infusion times2†
In the MARIPOSA-2 and PAPILLON trials, infusion times decreased over time.†
†Total infusion time is approximately 4 to 6 hours for day 1 and 6 to 8 hours for day 2.1
See infusion detailsProactive medications1
To help reduce the risk of select ARs
Dermatologic AR prophylaxis:
- Oral/topical antibiotics and ceramide-based moisturizer
- Advise patients to limit direct sun exposure‡
‡During and for 2 months after treatment.
See Proactive Therapy ManagementAR, adverse reaction.

RYBREVANT® Administration & Management Guide
Download a guide with comprehensive dosing, administration, and proactive therapy management details for RYBREVANT®-based regimens.
DownloadDosing Schedule
Recommended dosing schedule for RYBREVANT® + chemotherapy (Q3W)1
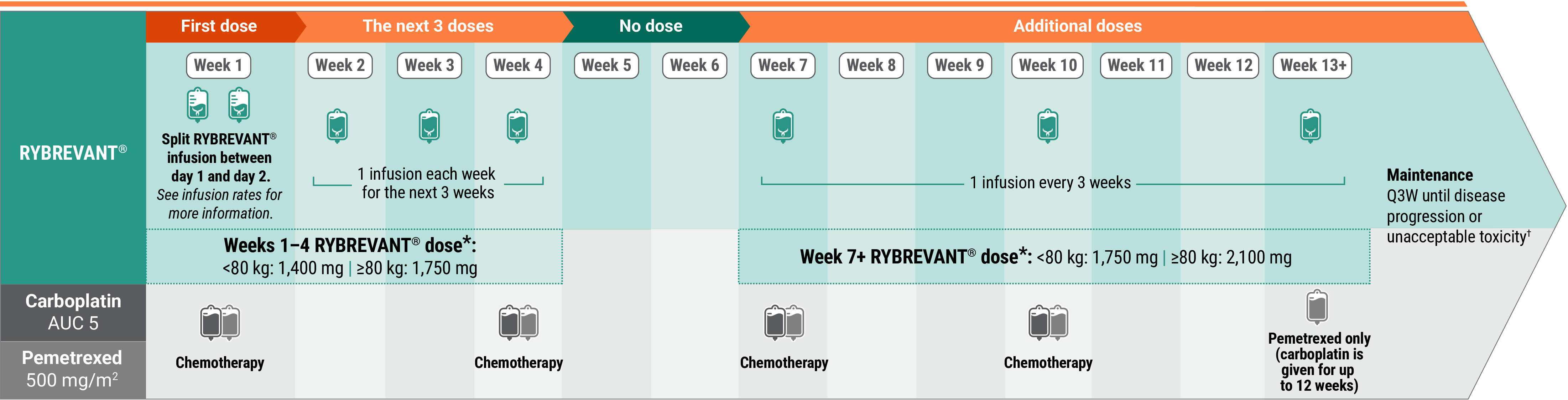
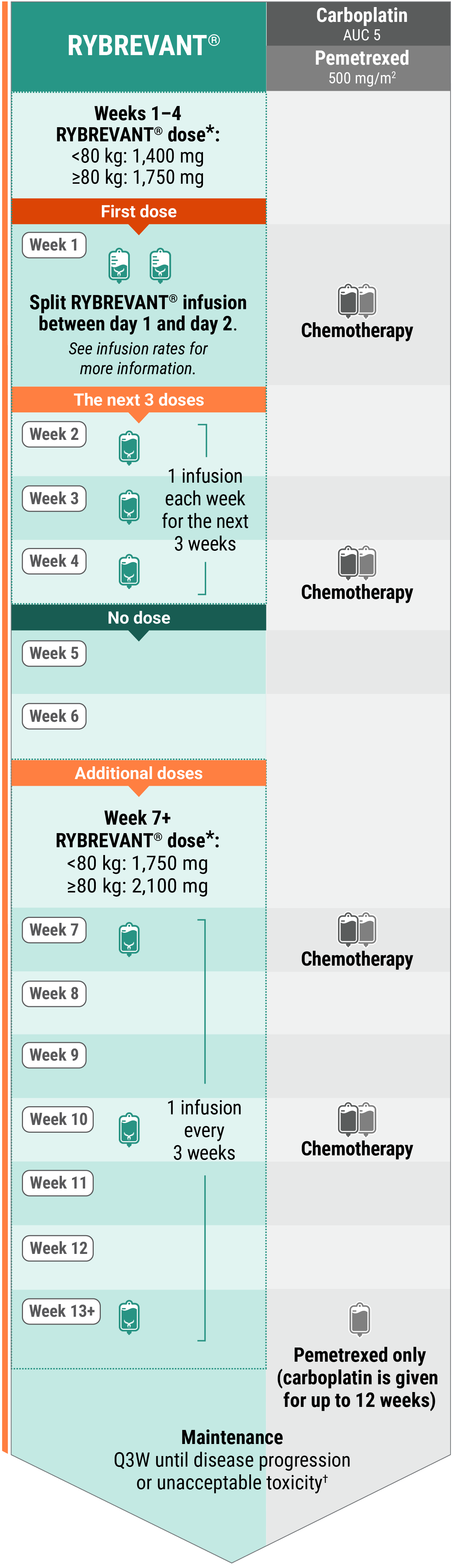
*Dose adjustments not required for subsequent body weight changes.
†This refers only to RYBREVANT® and pemetrexed. Carboplatin should only be administered every 3 weeks for up to 12 weeks.
RYBREVANT®
The recommended dosage is based on baseline body weight and can be administered as an intravenous infusion after dilution.1
With chemotherapy
Administer RYBREVANT® after chemotherapy. Administer in the following order: pemetrexed, carboplatin, and then RYBREVANT®.1
Refer to the full Prescribing Information for pemetrexed and carboplatin for the respective dosing information.
If switching from RYBREVANT® Q3W dosing to RYBREVANT FASPRO™ Q3W dosing, switch patients at their next scheduled dose on or after week 43
See Proactive Therapy Management to help reduce the risk of key ARs
Learn moreIn both the MARIPOSA-2 and PAPILLON trials, infusion times decreased over time with RYBREVANT®2
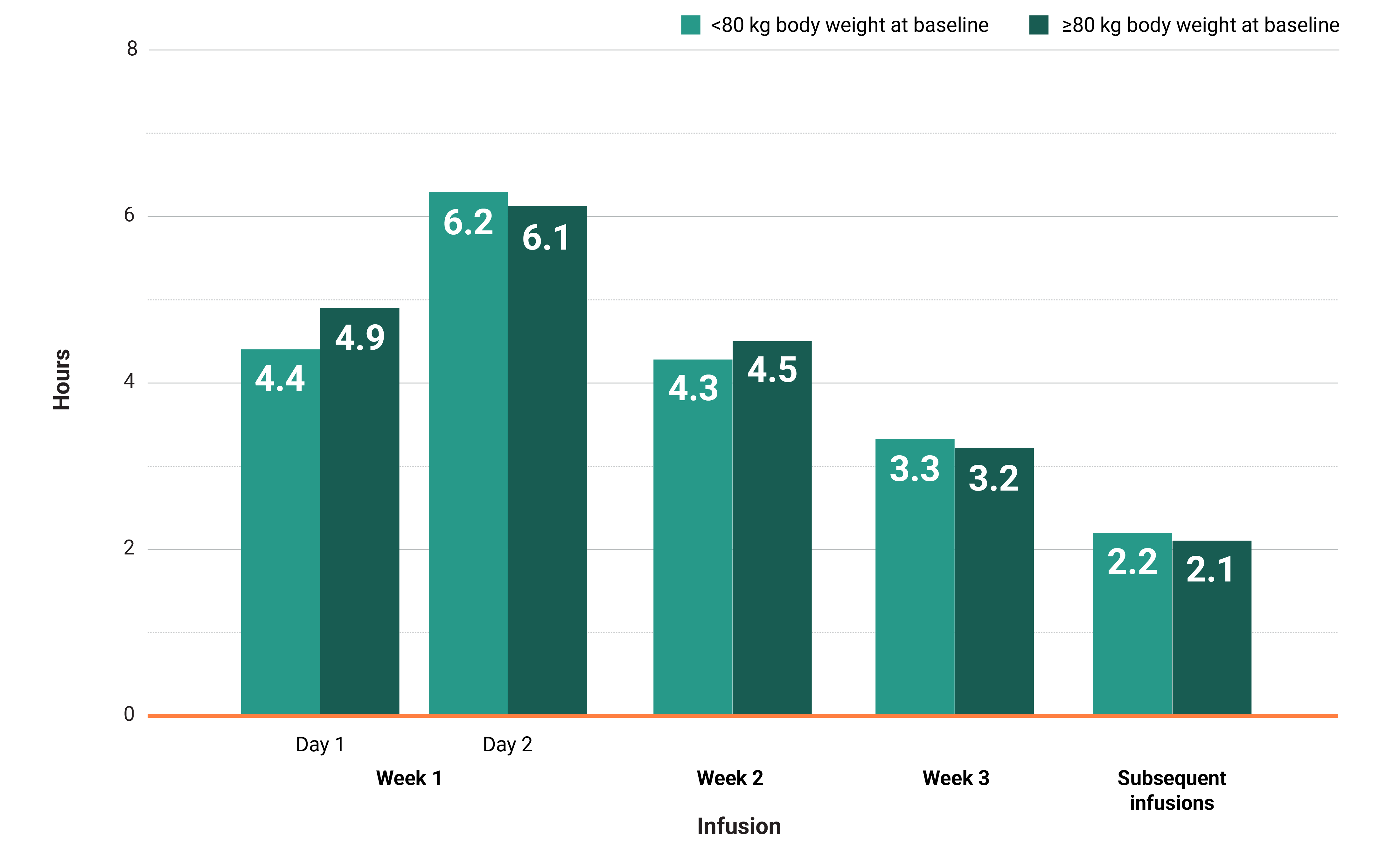
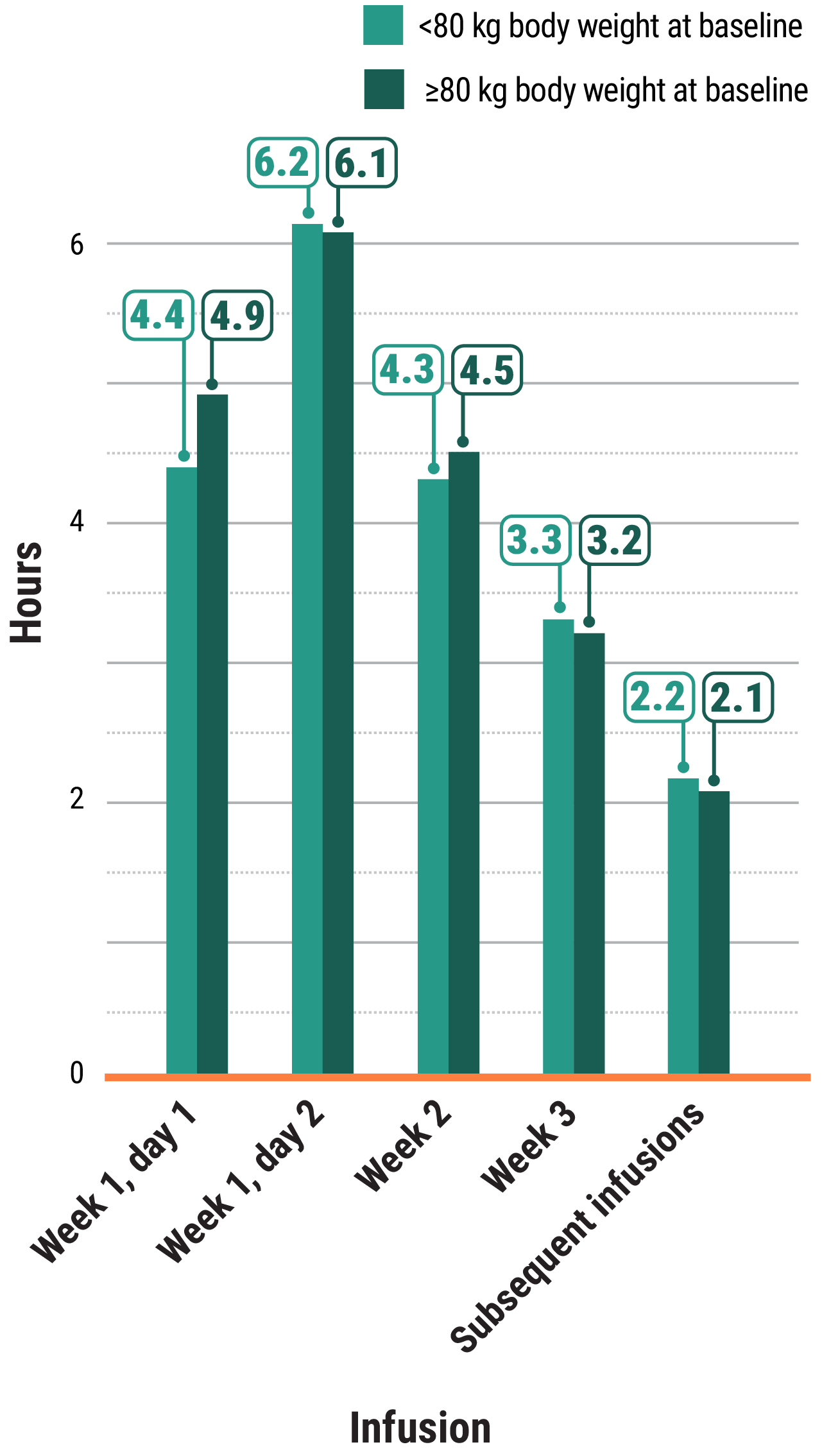
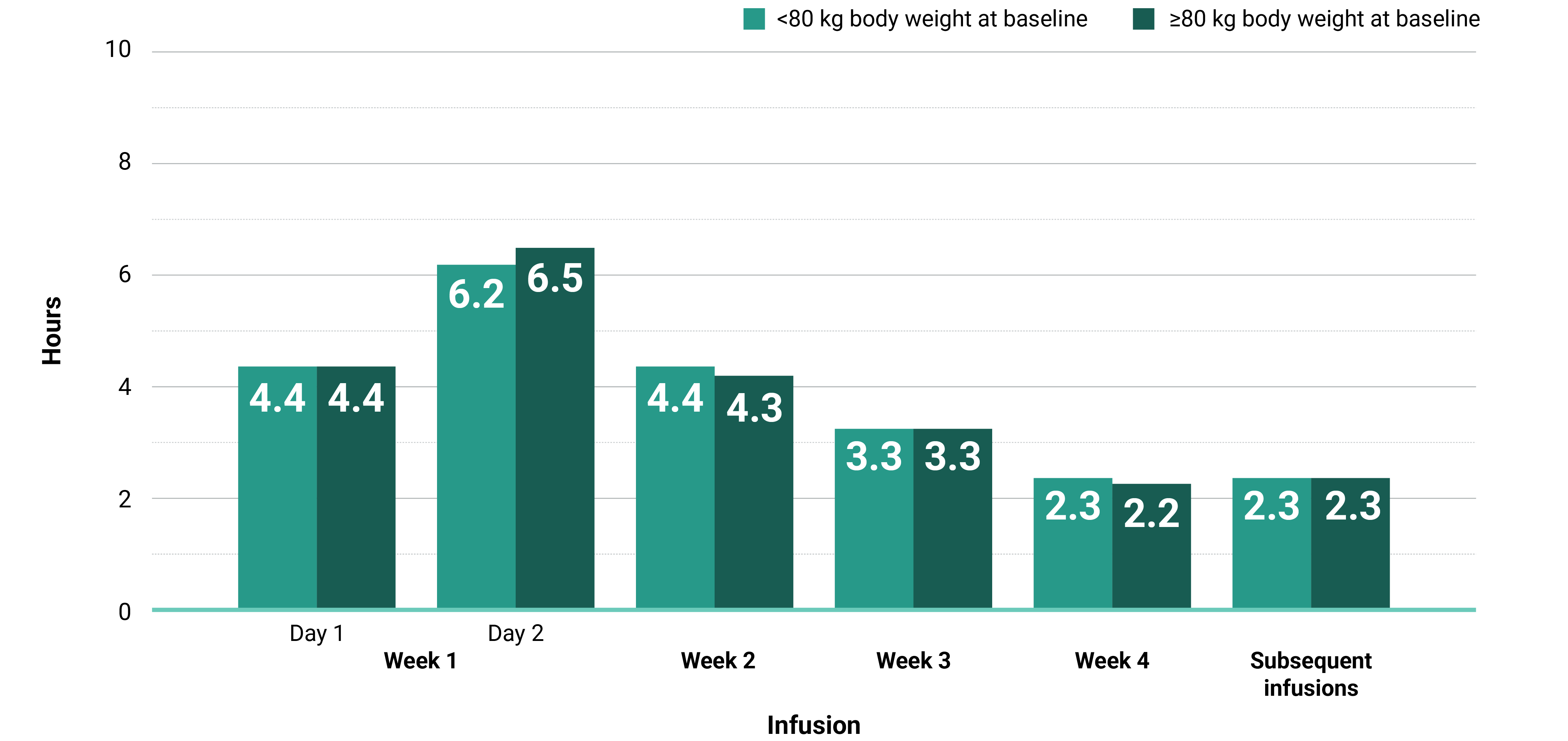
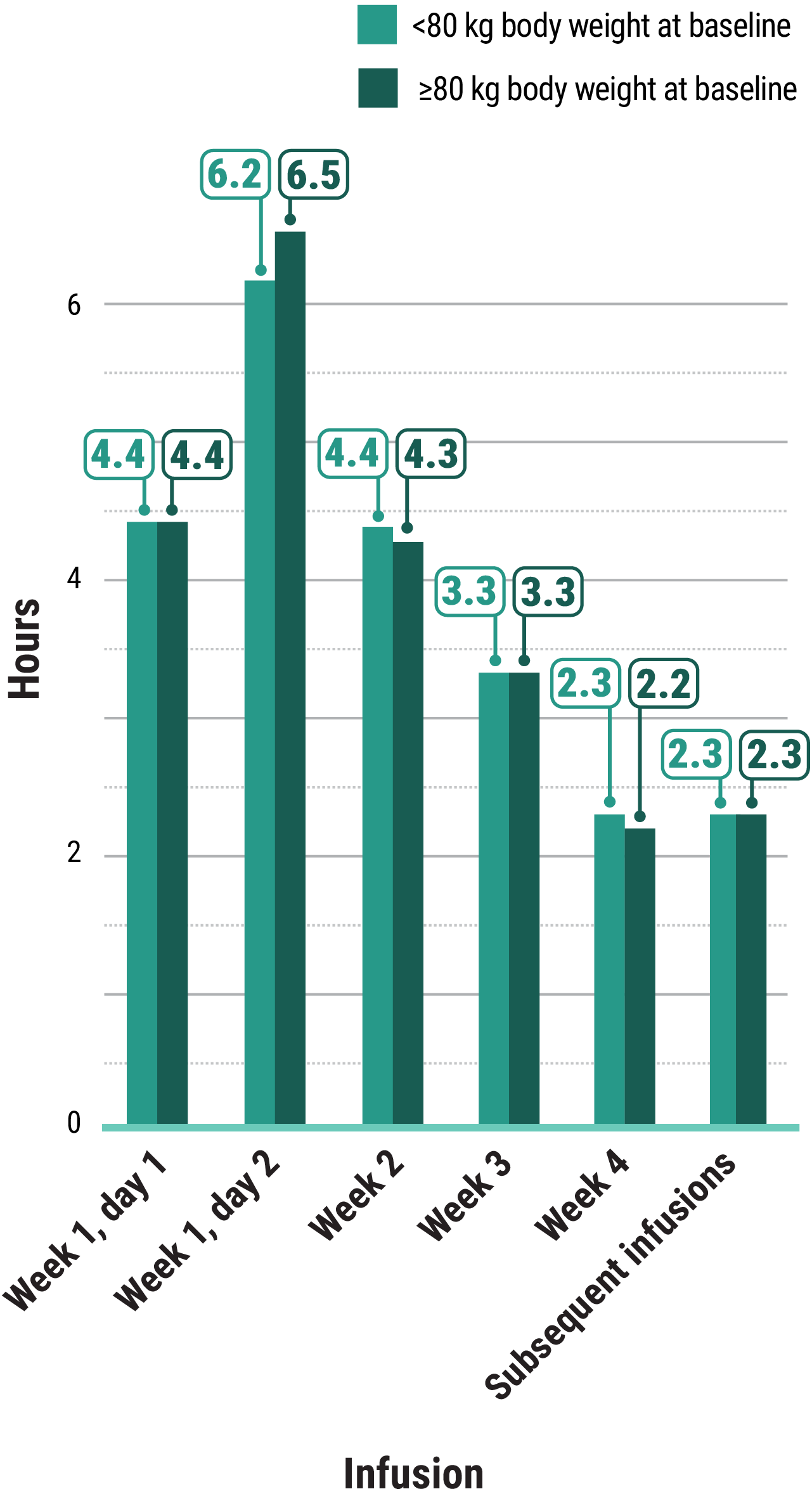
Total infusion time is approximately 4 to 6 hours for day 1 and 6 to 8 hours for day 2. Subsequent infusion time is approximately 2 hours.1
*Data reflect results from 3-week dosing in the MARIPOSA-2 trial.1
†Data reflect results from 3-week dosing in the PAPILLON trial.1
AUC, area under the curve; Q3W, once every 3 weeks.
Premedications
Premedications for RYBREVANT®1
IRR management
- Interrupt infusion if IRR is suspected. Reduce the infusion rate or permanently discontinue RYBREVANT® based on severity
- If an anaphylactic reaction occurs, permanently discontinue RYBREVANT®
Prophylactic medications1
Dermatologic AR prophylaxis
Prophylactic measures (eg, use of oral/topical antibiotics) are recommended to reduce the risk of dermatologic ARs. When initiating treatment with RYBREVANT®, ceramide-based moisturizer is recommended.
See additional strategies for dermatologic AR prophylaxisIRR, infusion-related reaction; IV, intravenous.
Preparation
Preparation1,3
Dilute and prepare RYBREVANT® for IV infusion before administration
STEP
1
During preparation and prior to administration, check the vial labels to ensure that the drug being prepared and administered is RYBREVANT® and not subcutaneous RYBREVANT FASPRO™
Check that the RYBREVANT® solution is colorless to pale yellow. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if discoloration or visible particles are present
STEP
2
Determine the dose required of RYBREVANT® based on patient’s baseline weight
- Each vial of RYBREVANT® contains 350 mg of amivantamab-vmjw
STEP
3
Withdraw and then discard a volume of either 5% dextrose injection or 0.9% sodium chloride injection from the 250 mL infusion bag equal to the volume of RYBREVANT® to be added (ie, discard 7 mL diluent from the infusion bag for each RYBREVANT® vial)
- Only use infusion bags made of PVC, PP, PE, or PP+PE
STEP
4
Withdraw 7 mL of RYBREVANT® from each vial and add it to the infusion bag. The final volume in the infusion bag should be 250 mL
- Discard any unused portion left in the vial
STEP
5
Gently invert the bag to mix the solution. Do not shake
STEP
6
Diluted solutions should be administered within 10 hours (including infusion time) at room temperature
PE, polyethylene; PP, polypropylene; PVC, polyvinyl chloride.
Administration
Administering RYBREVANT® infusions1
Administer premedications before each RYBREVANT® dose as recommended to reduce the risk of IRRs.
Administering RYBREVANT® in combination with carboplatin and pemetrexed1
- Administer RYBREVANT® in combination with carboplatin and pemetrexed infusions every 3 weeks intravenously until disease progression or unacceptable toxicity according to the infusion rates. Administer the pemetrexed infusion first, carboplatin infusion second, and the RYBREVANT® infusion last
- Administer the diluted RYBREVANT® solution by IV infusion using an infusion set fitted with a flow regulator and with an in-line, sterile, non-pyrogenic, low protein-binding PES filter (pore size 0.2 micrometer). Administration sets must be made of PU, PBD, PVC, PP, or PE
- The administration set with filter must be primed with either 5% dextrose injection or 0.9% sodium chloride injection prior to the initiation of each RYBREVANT® infusion
- Do not infuse RYBREVANT® concomitantly in the same IV line with other agents
- Administer RYBREVANT® via a peripheral line on week 1 and week 2 to reduce the risk of IRRs during initial treatment. RYBREVANT® may be administered via a central line for subsequent weeks
- For the initial infusion, prepare RYBREVANT® as close to administration time as possible to allow for the possibility of extended infusion time in the event of an IRR
PBD, polybutadiene; PES, polyethersulfone; PU, polyurethane.
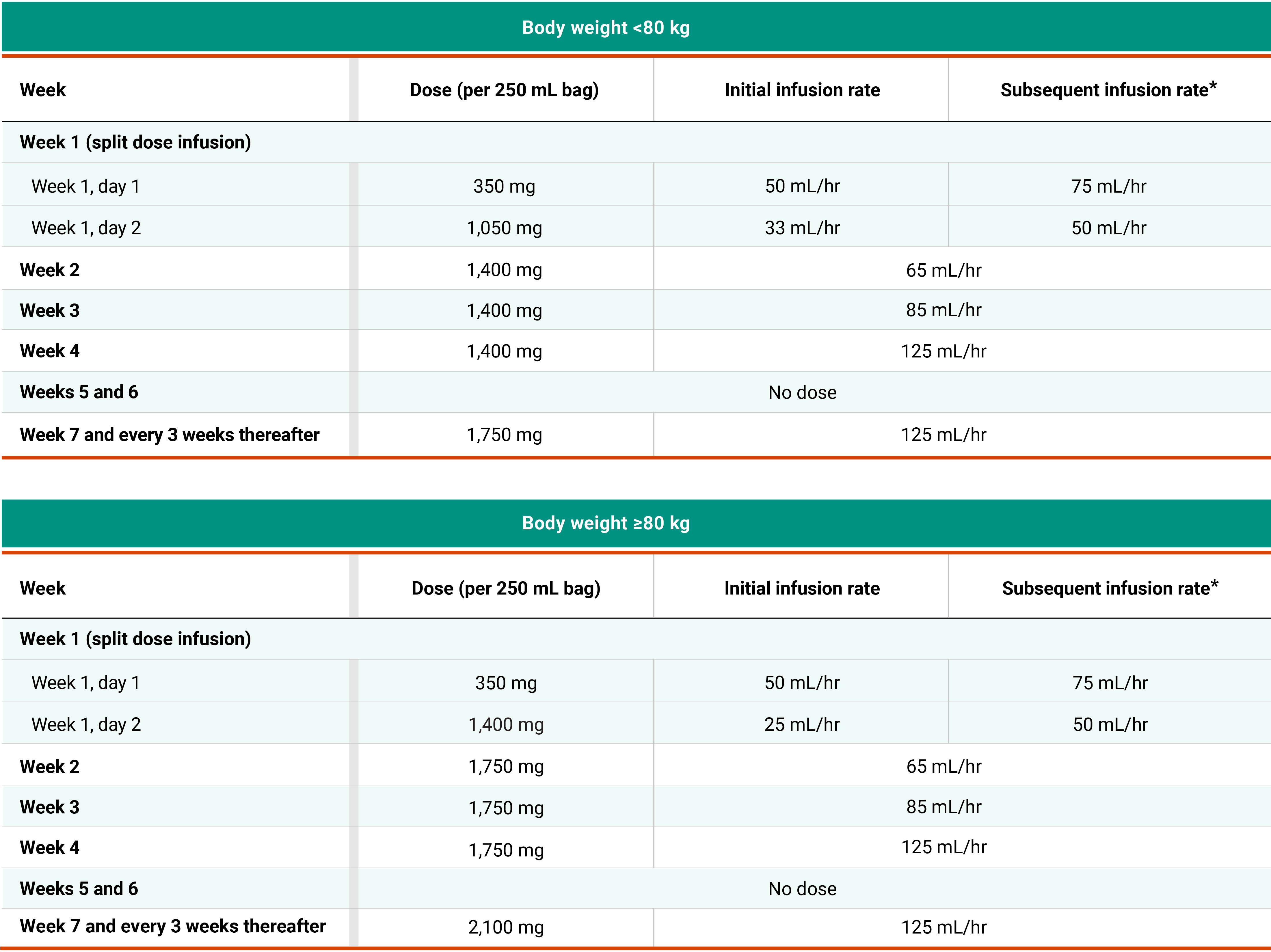
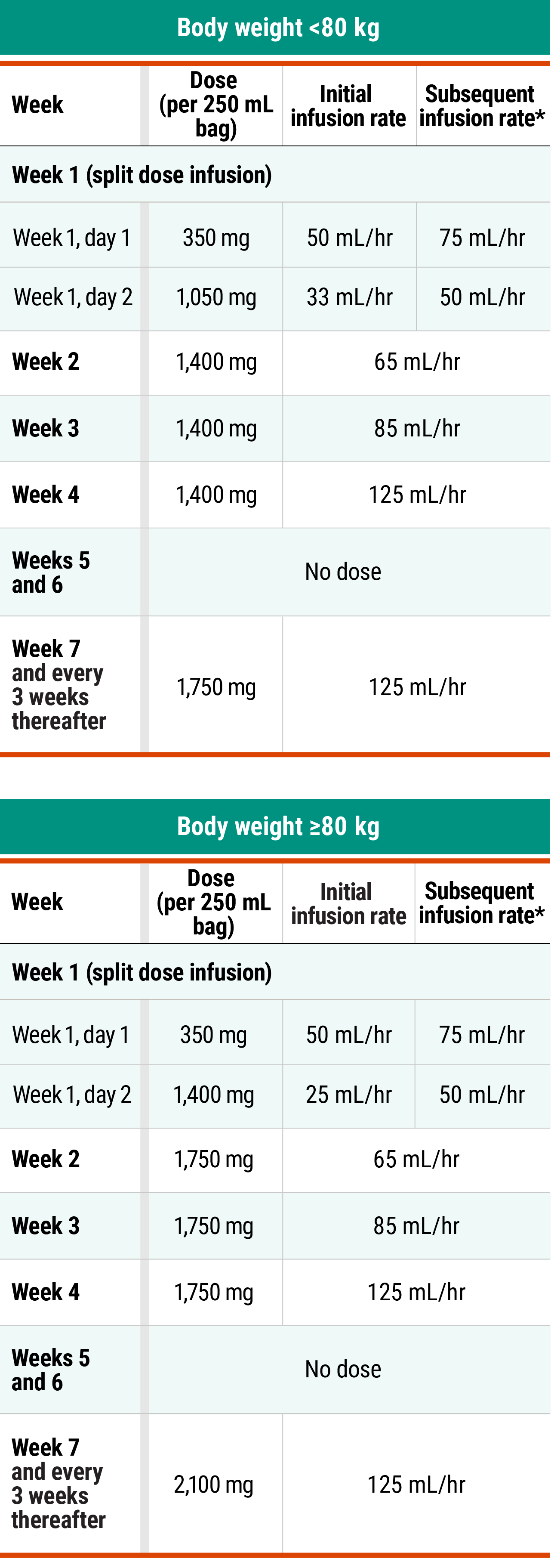
*In the absence of IRRs, increase the initial infusion rate to the subsequent infusion rate after 2 hours based on patient tolerance. Total infusion time is approximately 4 to 6 hours for day 1 and 6 to 8 hours for day 2. Subsequent infusion time is approximately 2 hours.
References:
- RYBREVANT® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Data on file. Janssen Biotech, Inc.
- RYBREVANT FASPRO™ [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.

