Dosing Schedule
Recommended dosing schedule for RYBREVANT® + LAZCLUZE™1,2

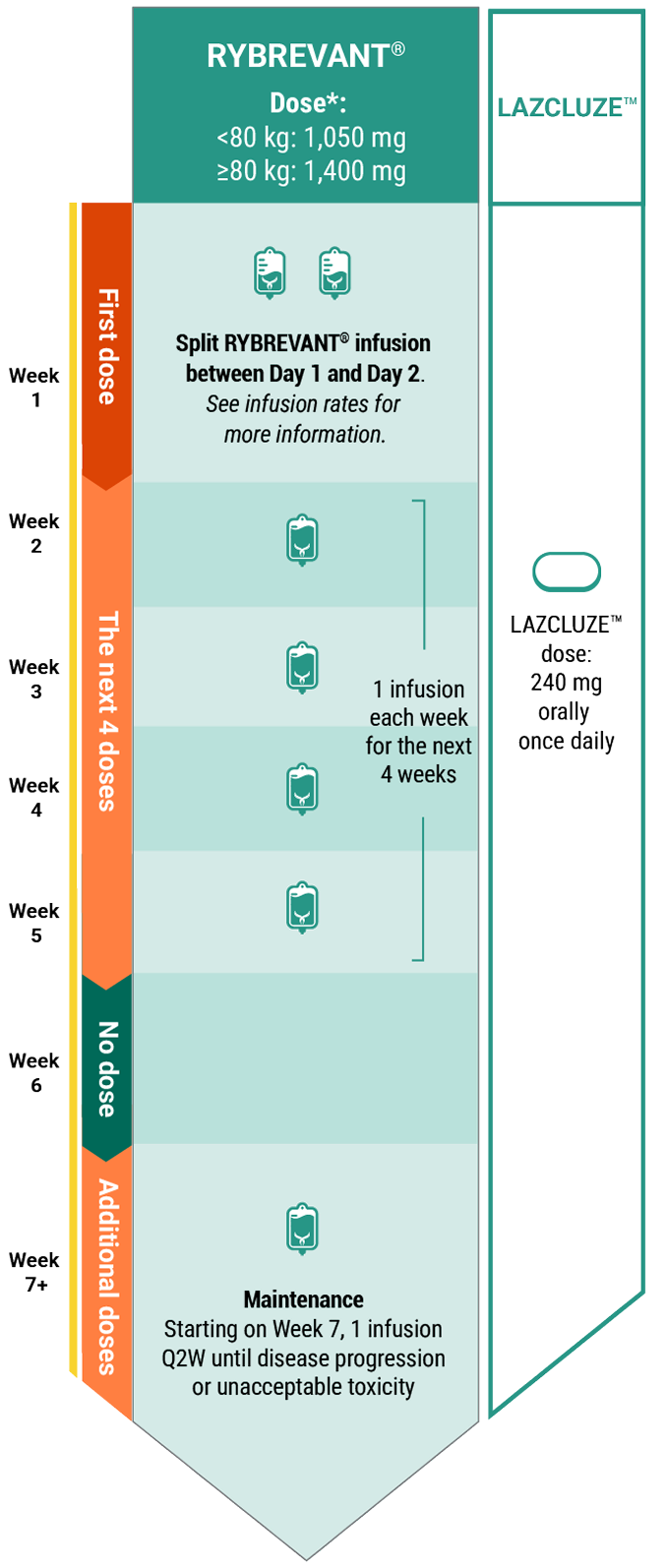
*Dose adjustments not required for subsequent body weight changes.
The recommended dosage of RYBREVANT® is based on baseline body weight and can be administered as an intravenous infusion after dilution.1
With LAZCLUZE™
When given in combination with LAZCLUZE™, administer LAZCLUZE™ any time before RYBREVANT® when given on the same day.1
Refer to the full Prescribing Information for LAZCLUZE™ for recommended LAZCLUZE™ dosing information.
See Proactive Therapy Management to help reduce the risk of key ARs
Learn moreIn the MARIPOSA trial, infusion times decreased over time with RYBREVANT®3
Clinical trial median infusion times by hours*
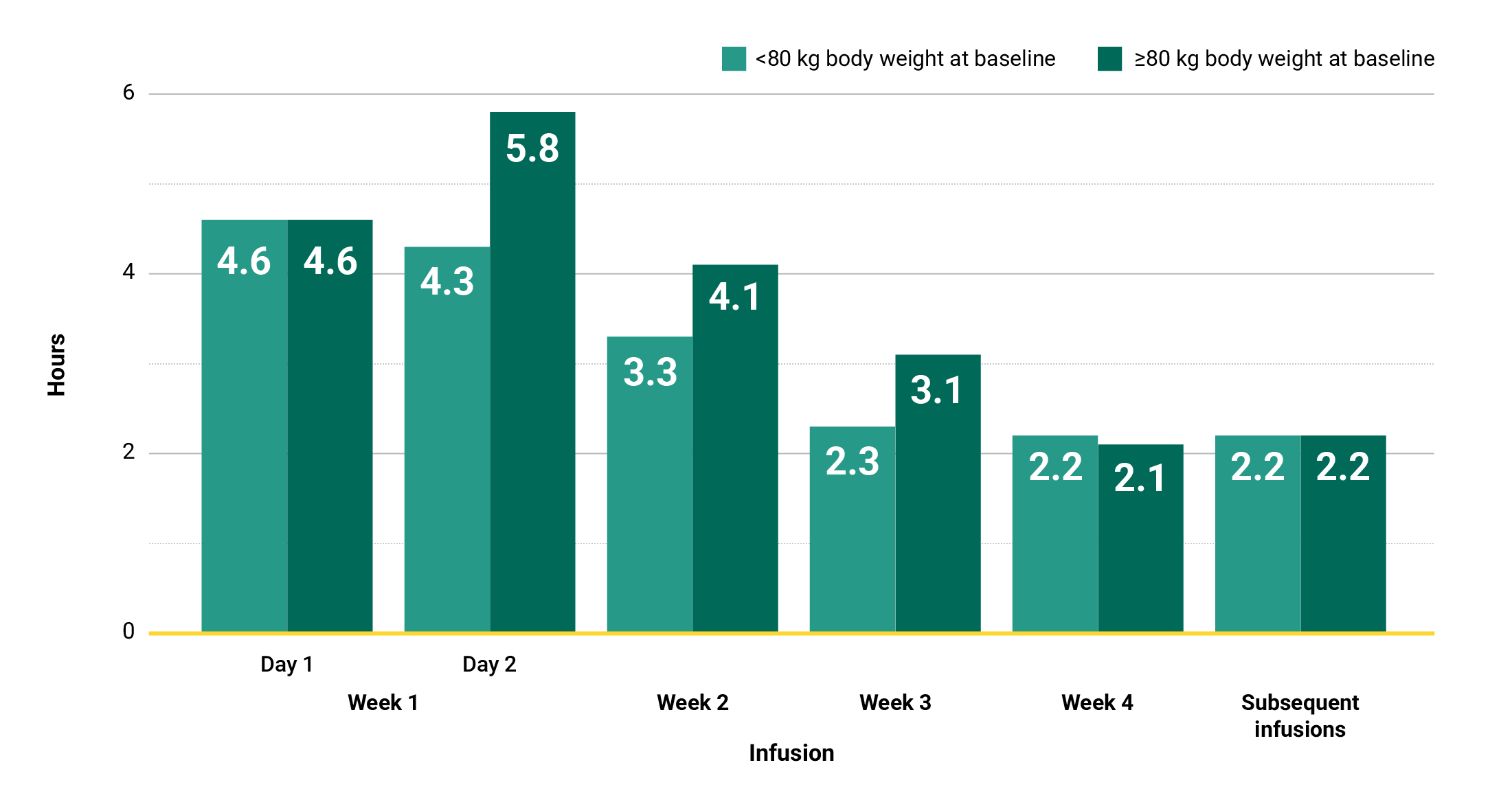
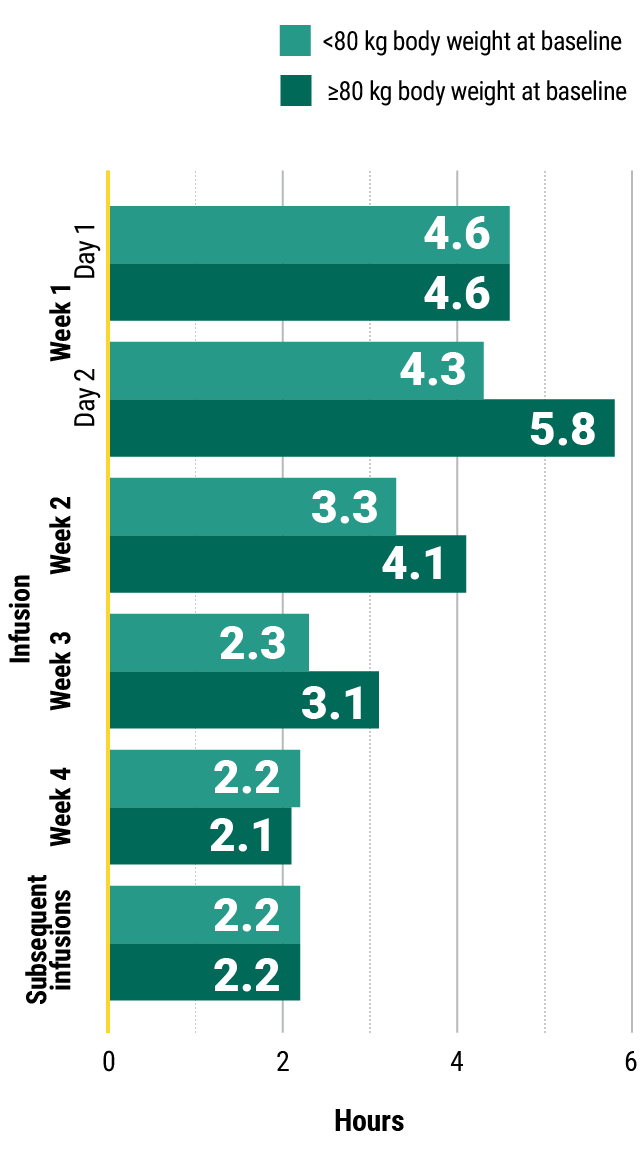
Total infusion time is approximately 4 to 6 hours for Day 1 and 6 to 8 hours for Day 2. Subsequent infusion time is approximately 2 hours.1
*Data reflect results from 2-week dosing in the MARIPOSA trial.1
AR, adverse reaction; Q2W, once every 2 weeks.
Preparation
Preparation of RYBREVANT®1
Dilute and prepare RYBREVANT® for intravenous infusion before administration
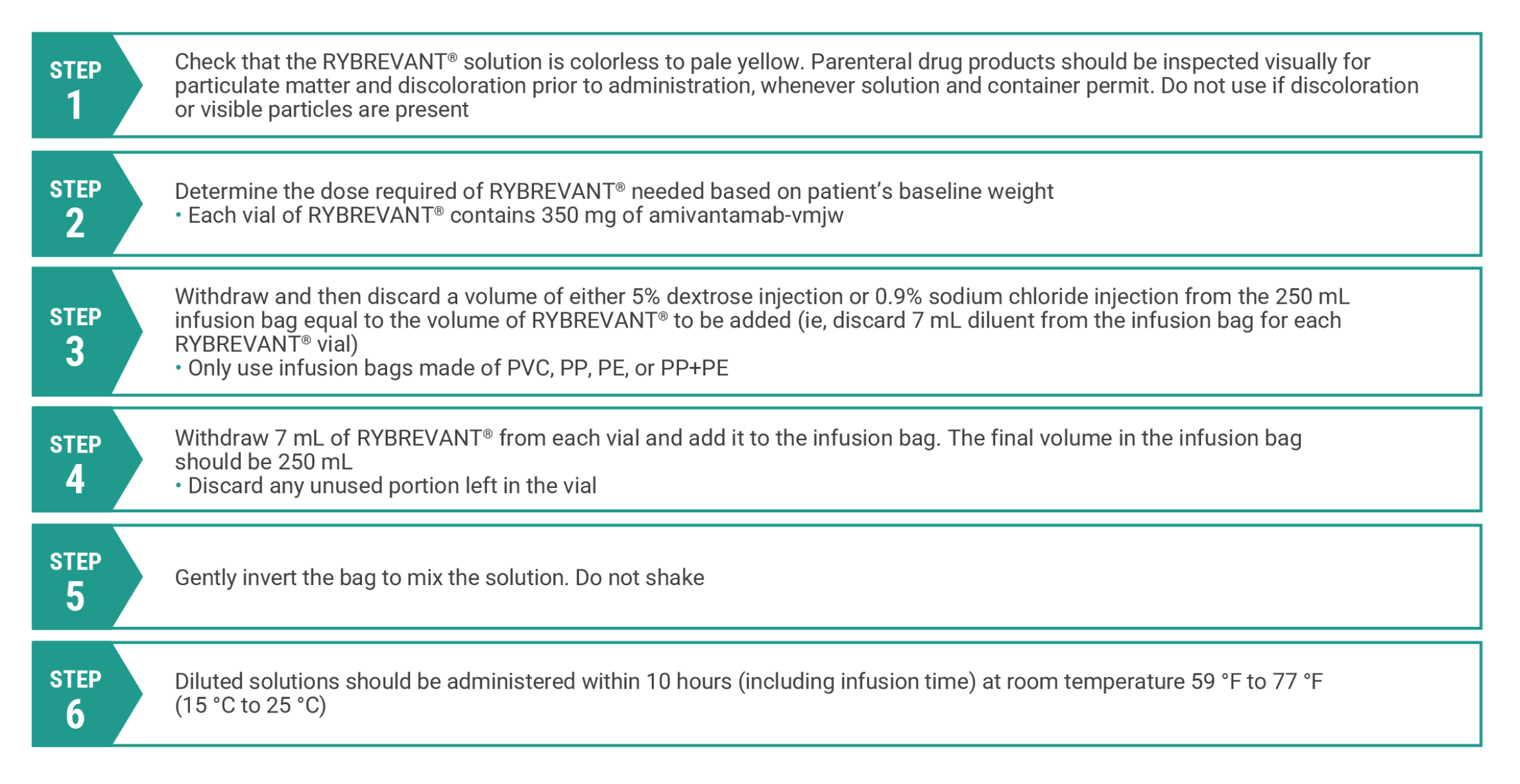

PE, polyethylene; PP, polypropylene; PVC, polyvinylchloride.
Administration
Administration for RYBREVANT®1
- Administer the diluted solution by intravenous infusion using an infusion set fitted with a flow regulator and with an in-line, sterile, non-pyrogenic, low protein-binding PES filter (pore size 0.2 micrometer)
- Administration sets must be made of PU, PBD, PVC, PP, or PE
- The administration set with filter must be primed with either 5% dextrose injection or 0.9% sodium chloride injection prior to the initiation of each RYBREVANT® infusion
- Do not infuse RYBREVANT® concomitantly in the same intravenous line with other agents
RYBREVANT® + LAZCLUZE™ or RYBREVANT® as a single agent1
- Administer RYBREVANT® as a single agent infusion every 2 weeks intravenously until disease progression or unacceptable toxicity according to the infusion rates
- Administer RYBREVANT® via a peripheral line on Week 1 and Week 2 to reduce the risk of IRRs during initial treatment
- RYBREVANT® may be administered via a central line for subsequent weeks
- For the initial infusion, prepare RYBREVANT® as close to administration time as possible to allow for the possibility of extended infusion time in the event of an IRR
- When given in combination with LAZCLUZE™, administer RYBREVANT® any time after LAZCLUZE™ when given on the same day
Dosing Information for LAZCLUZE™ when given in combination with RYBREVANT®2
- Swallow LAZCLUZE™ tablets whole (with or without food). Do not crush, split, or chew. Continue treatment until disease progression or unacceptable toxicity
- If a patient misses a dose of LAZCLUZE™ within 12 hours, instruct the patient to take the missed dose. If more than 12 hours have passed since the dose was to be given, instruct the patient to take the next dose at its scheduled time
- If vomiting occurs any time after taking LAZCLUZE™, instruct the patient to take the next dose at its next regularly scheduled time
Drug interactions with LAZCLUZE™2
Avoid concomitant use of LAZCLUZE™ with strong and moderate CYP3A4 inducers. Consider an alternate concomitant medication with no potential to induce CYP3A4.
Monitor for adverse reactions associated with a CYP3A4 or BCRP substrate where minimal concentration changes may lead to serious adverse reactions, as recommended in the approved product labeling for the CYP3A4 or BCRP substrate.
Please see the full Prescribing Information for LAZCLUZE™ for information regarding dosing and drug interactions.
BCRP, breast cancer resistance protein; CYP3A4, cytochrome P450 3A4; IRR, infusion-related reaction; PBD, polybutadiene; PES, polyethersulfone; PU, polyurethane.
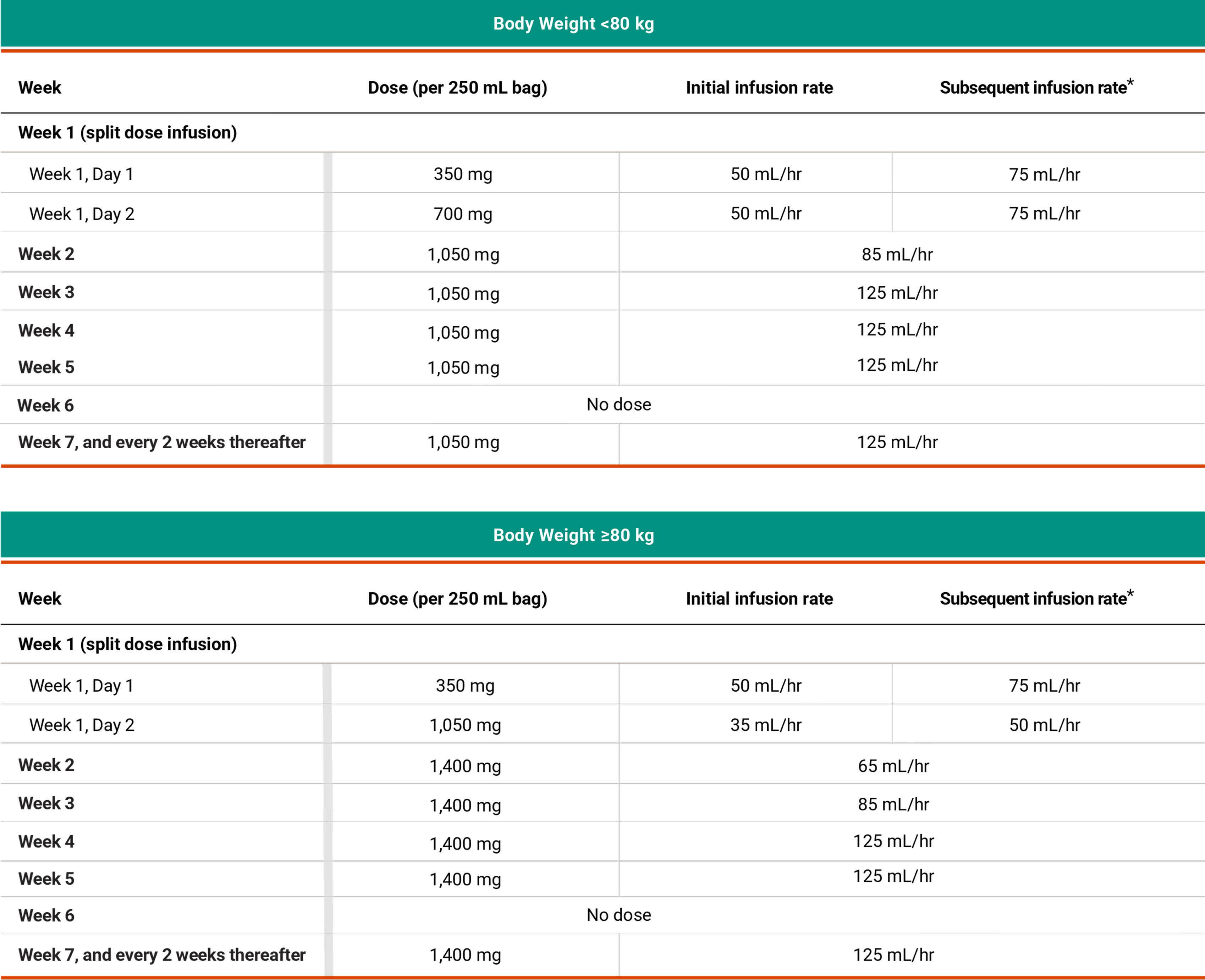
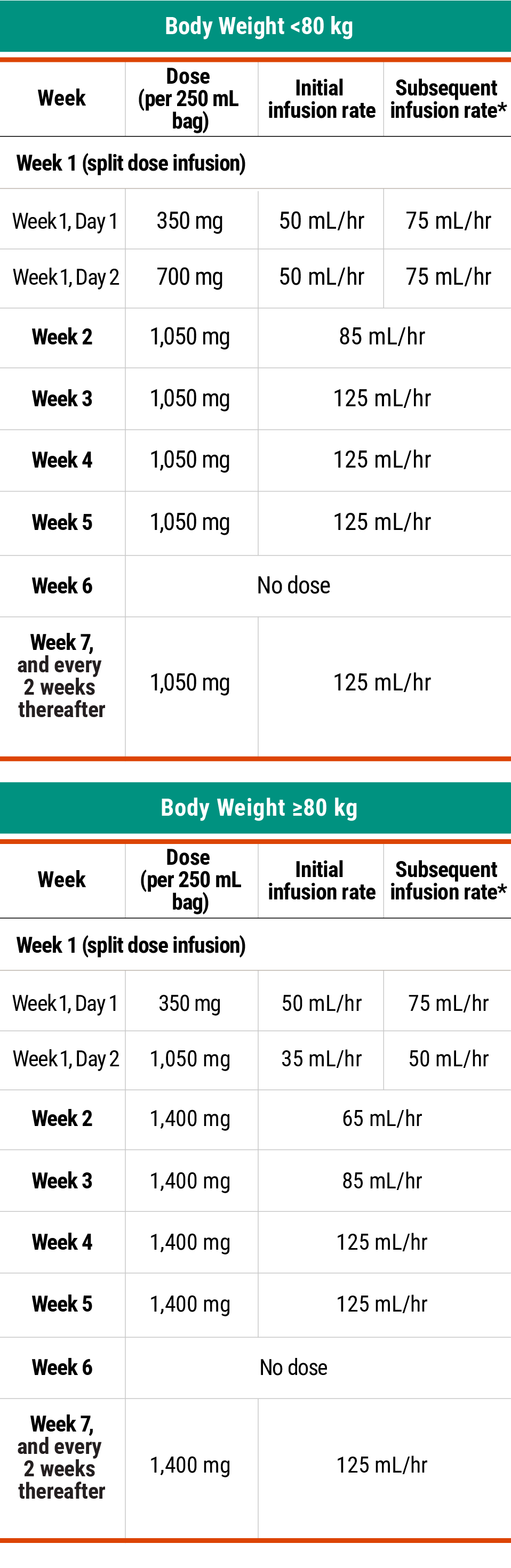
*In the absence of IRRs, increase the initial infusion rate to the subsequent infusion rate after 2 hours based on patient tolerance. Total infusion time is approximately 4 to 6 hours for Day 1 and 6 to 8 hours for Day 2. Subsequent infusion time is approximately 2 hours.
References:
- RYBREVANT® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- LAZCLUZE™ [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Data on file. Janssen Biotech, Inc.

