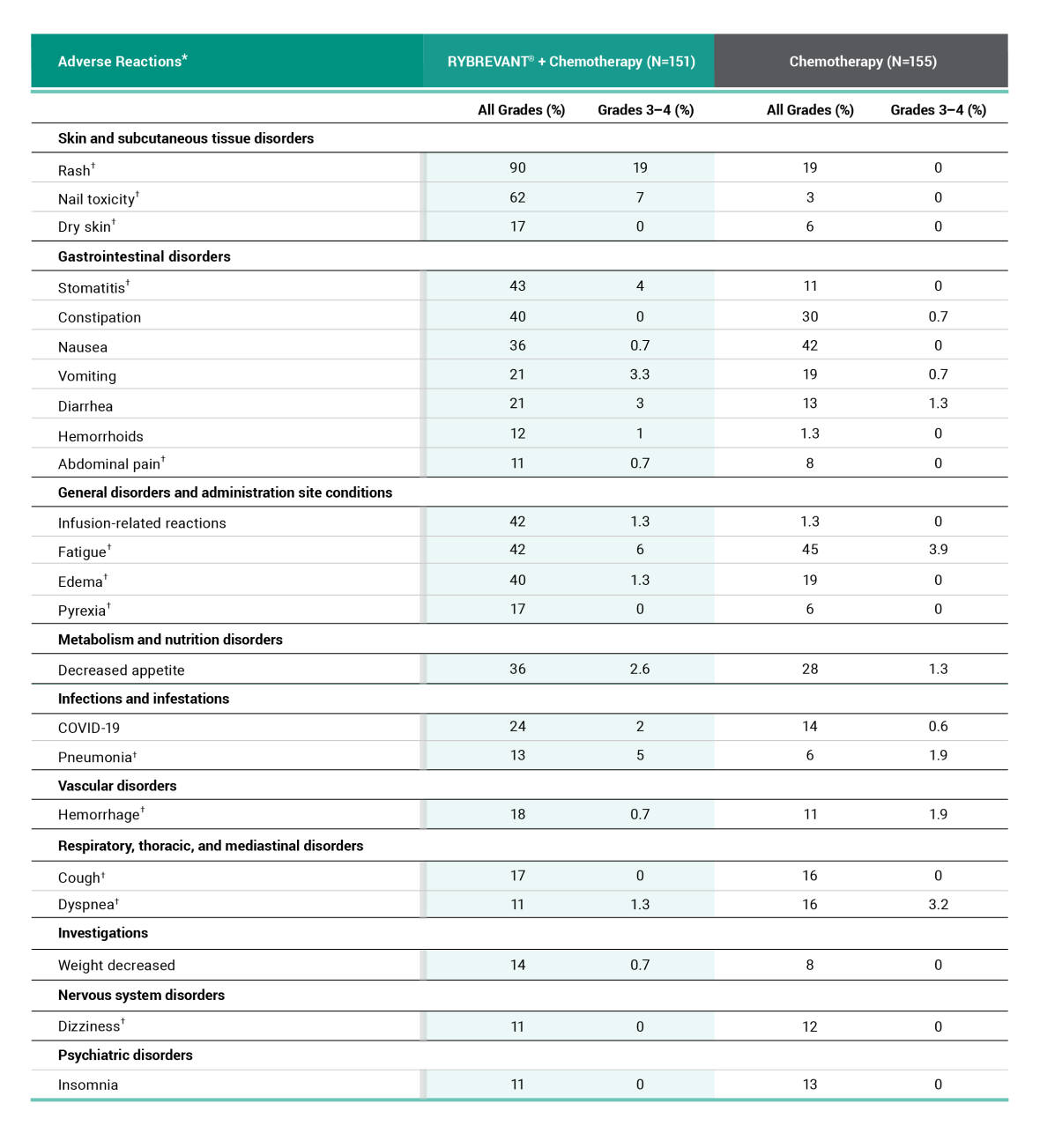
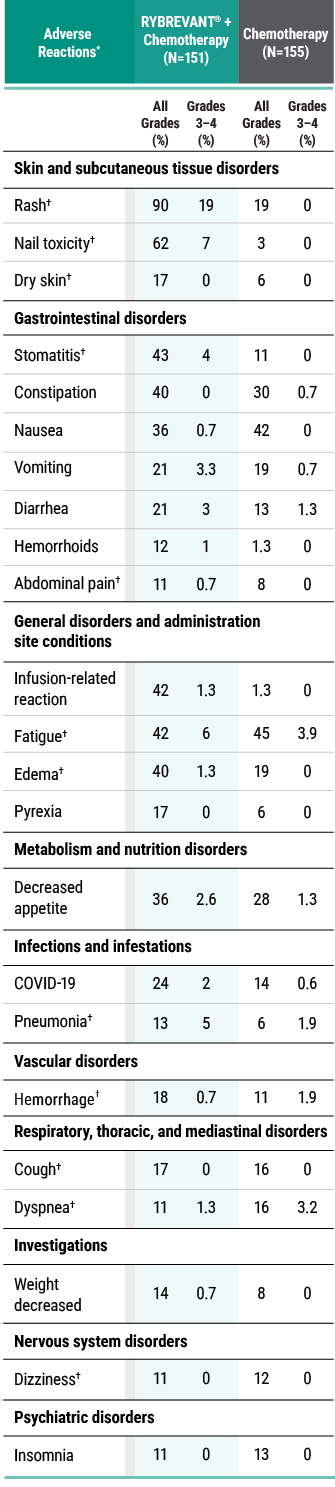
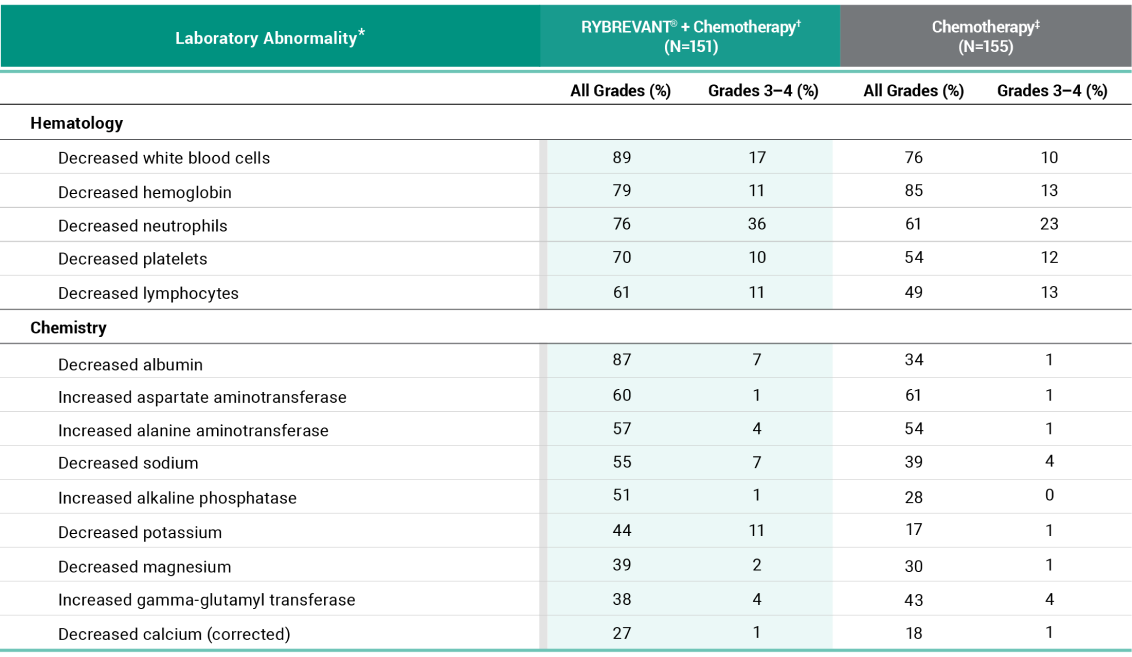
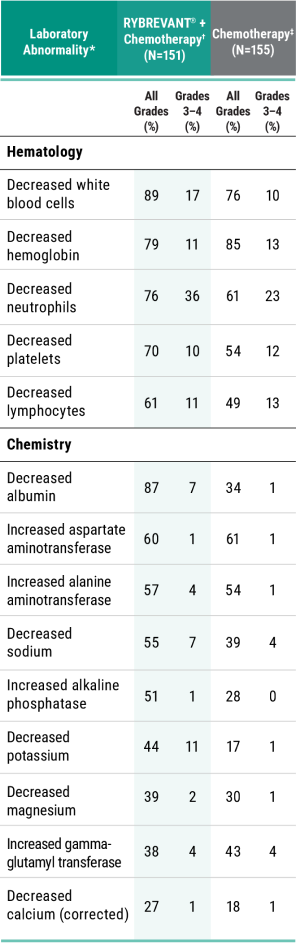
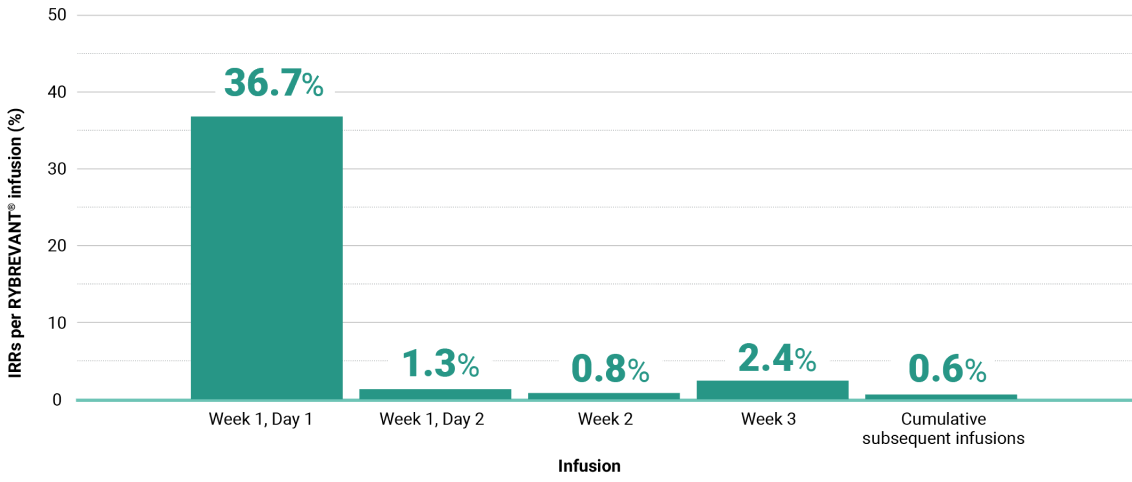
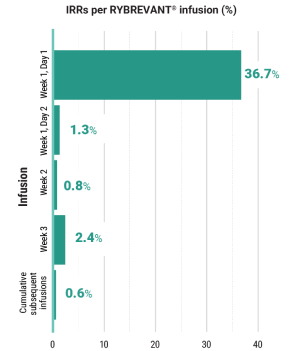
Primary Endpoint
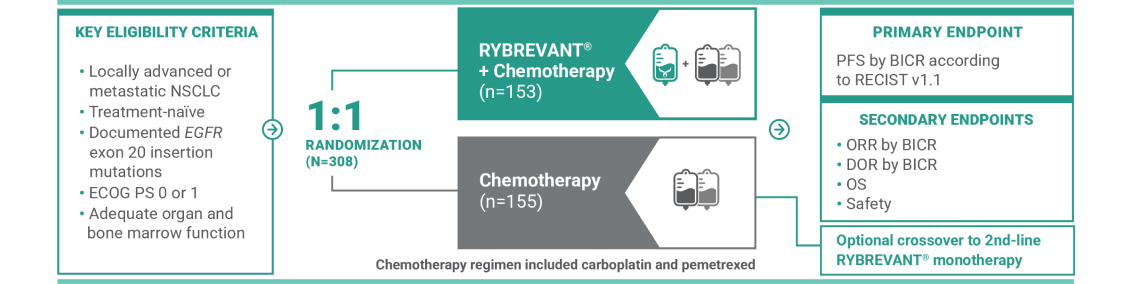
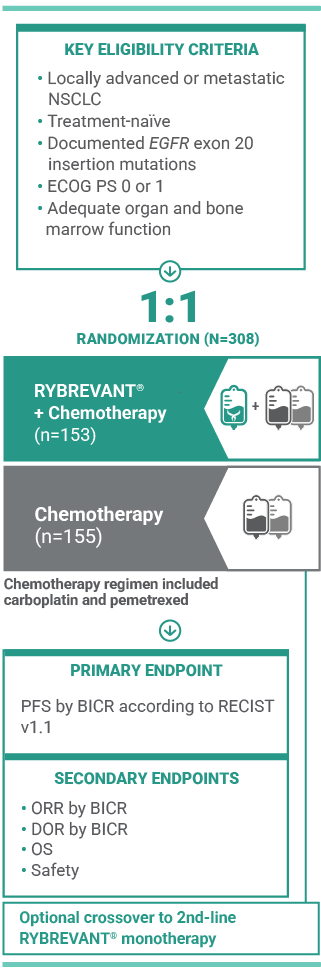
For first-line treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations
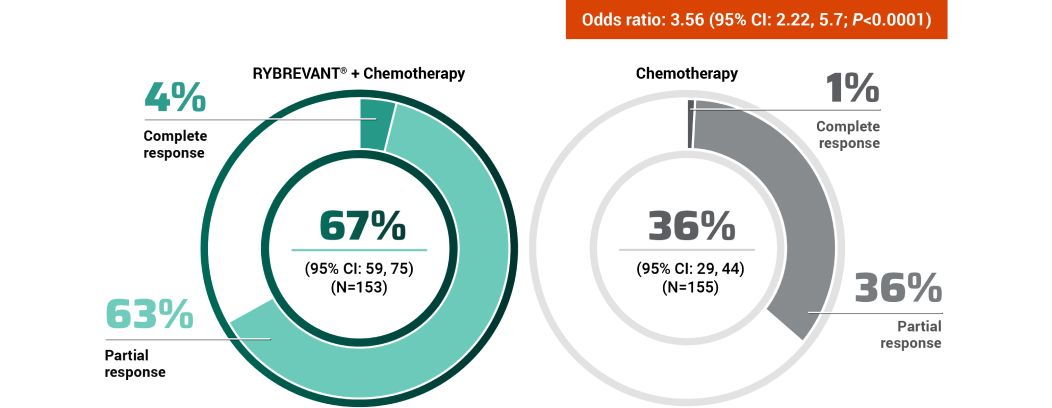
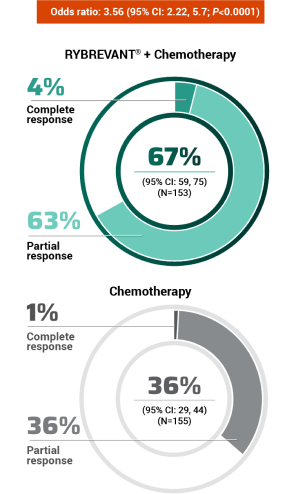
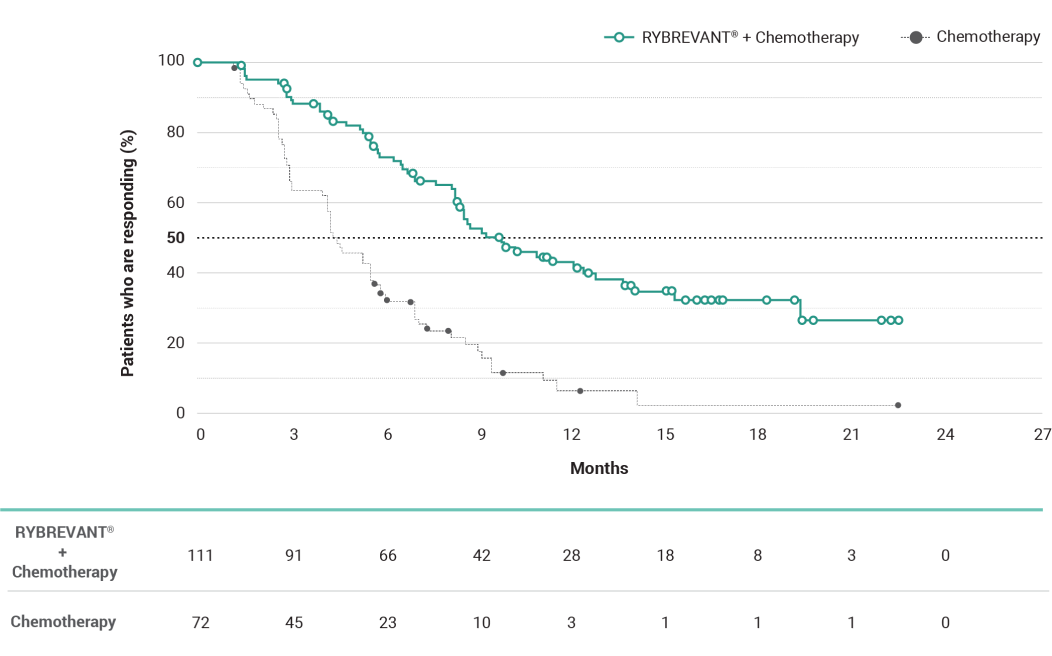
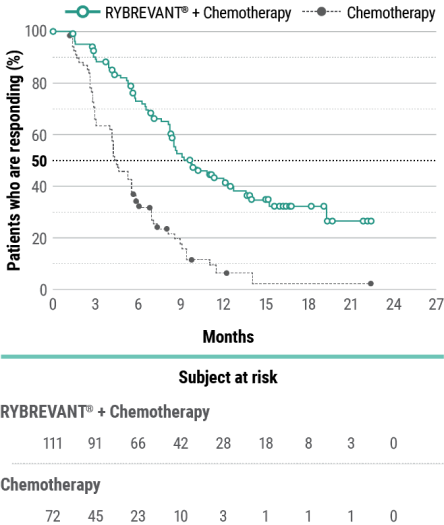
The first and only targeted therapy to show statistically significant PFS improvements1
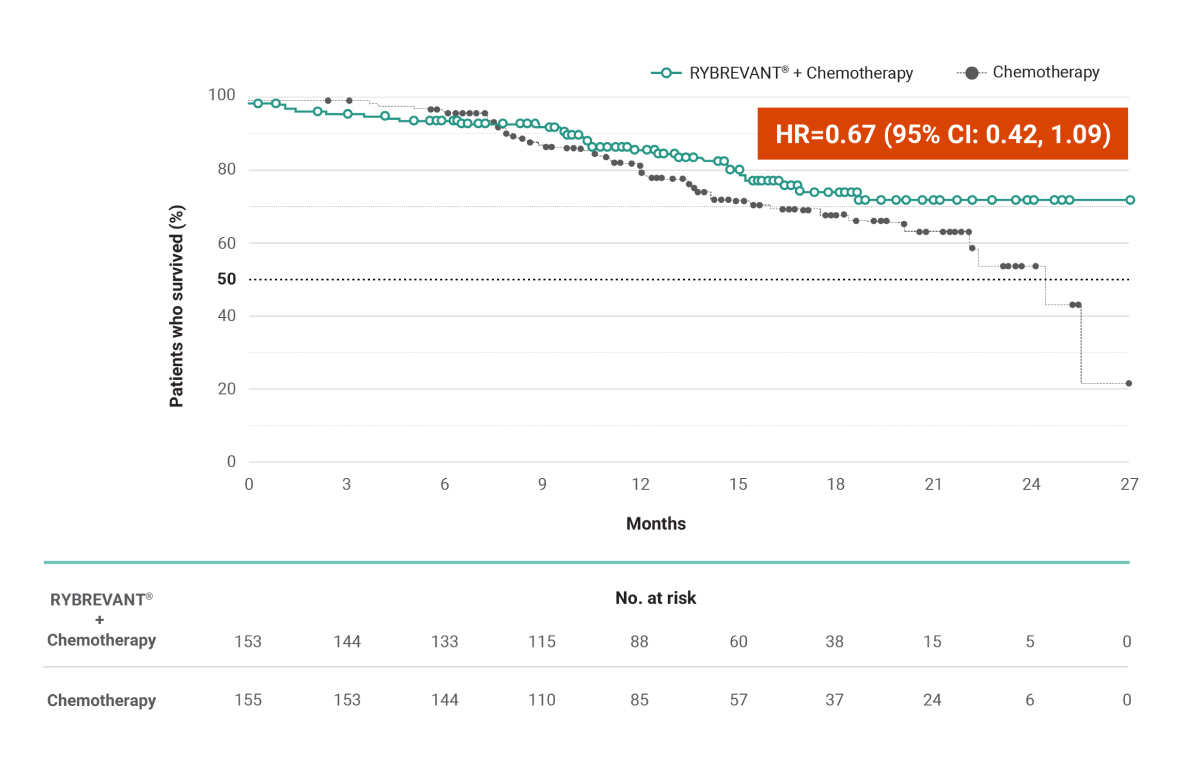
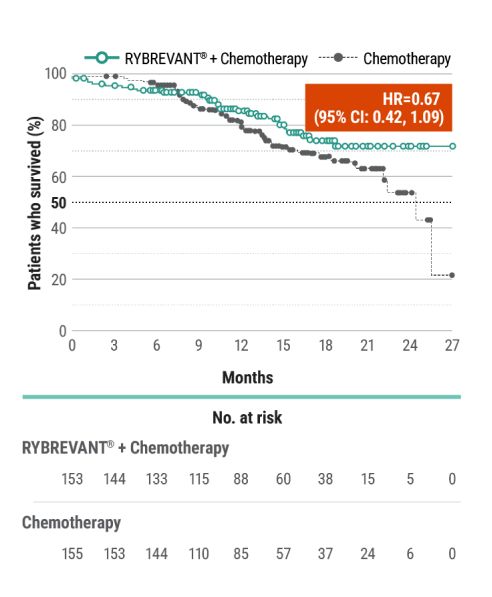
RYBREVANT® + chemotherapy demonstrated a statistically significant reduction in the risk of progression or death by 60% vs chemotherapy alone, as assessed by BICR1,2
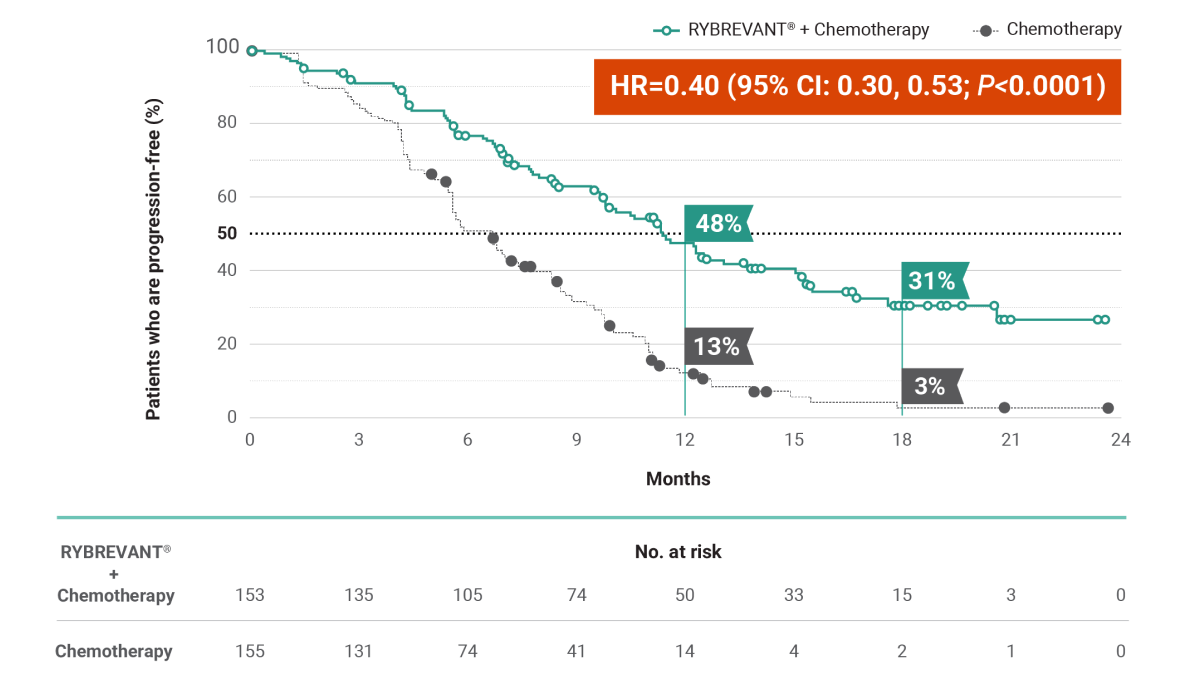
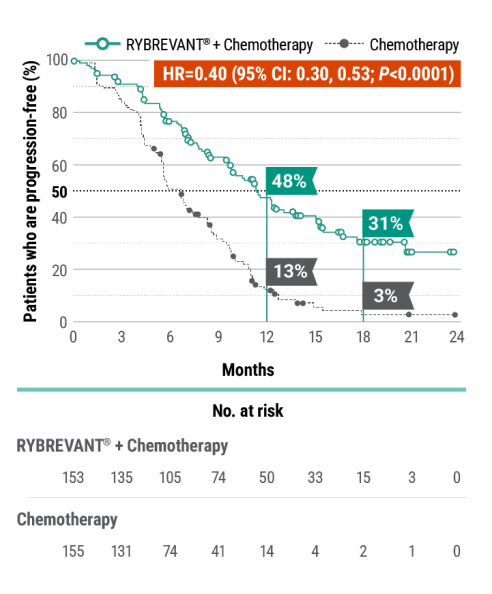
~2X mPFS: 11.4 months (95% CI: 9.8, 13.7) with RYBREVANT® + chemotherapy vs 6.7 months (95% CI: 5.6, 7.3) with chemotherapy alone1
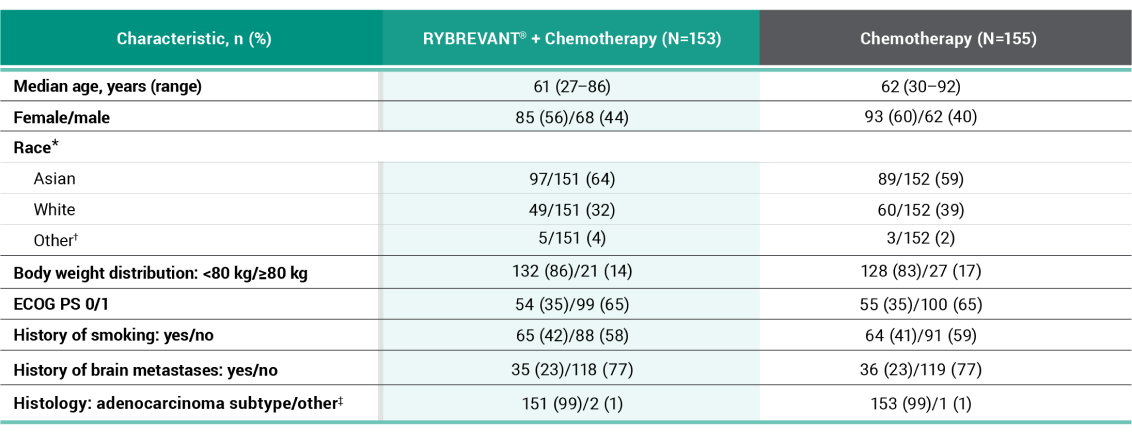
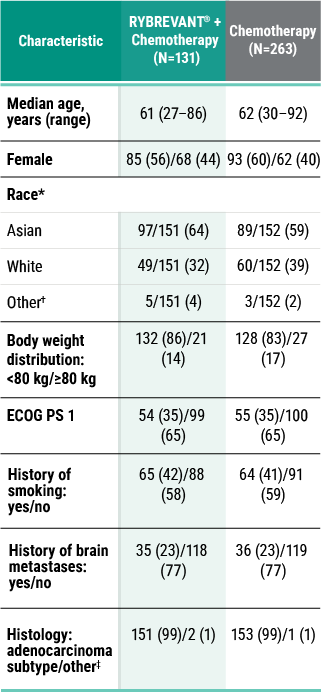
PFS results in prespecified subgroups2
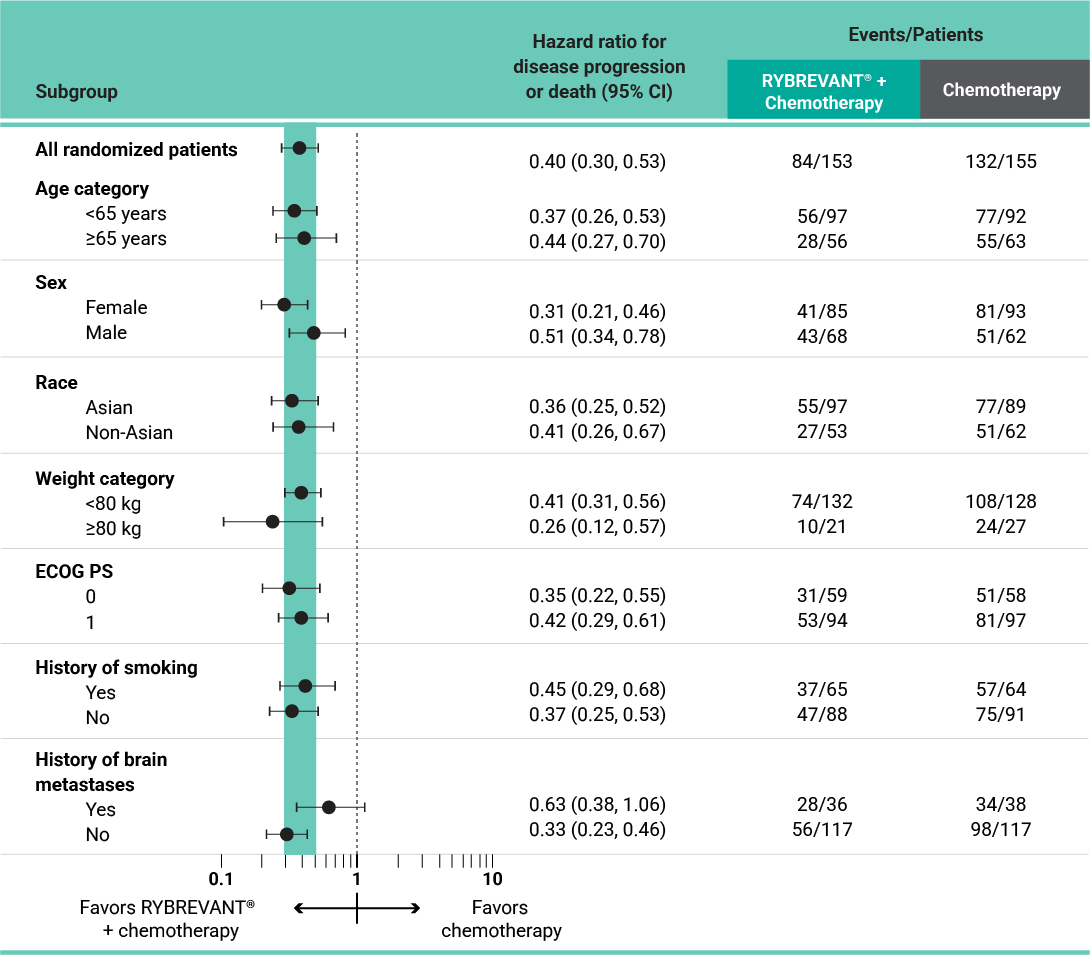
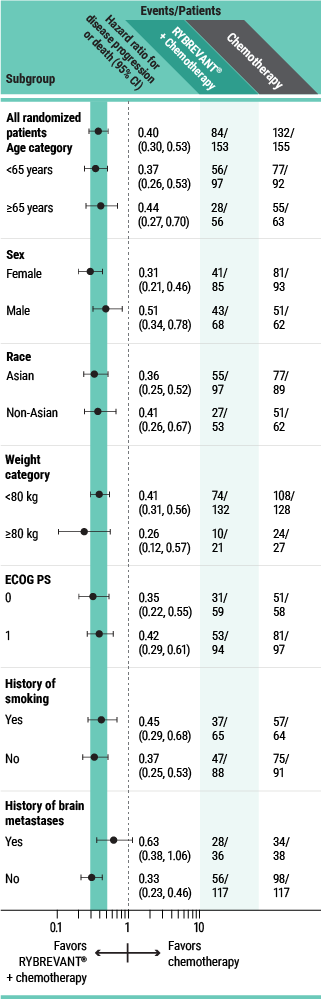
This was a prespecified analysis and was not powered to show statistical significance.
RYBREVANT® is also approved for patients with EGFR exon 20 insertion mutations whose disease has progressed on or after platinum-based chemotherapy1
Learn moreBICR, blinded independent central review; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; HR, hazard ratio; mPFS, median progression-free survival; NSCLC, non–small cell lung cancer; PFS, progression-free survival.