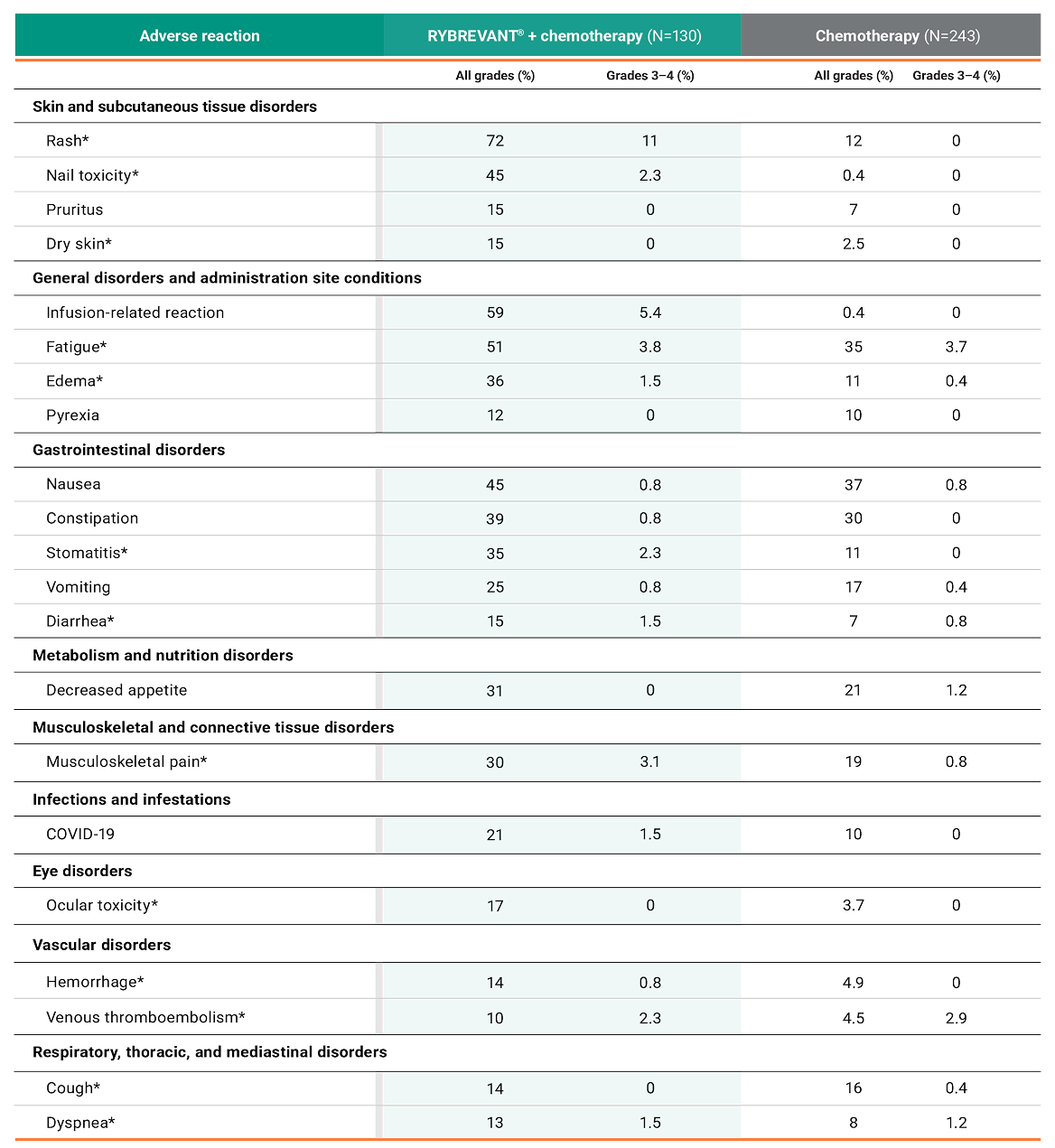
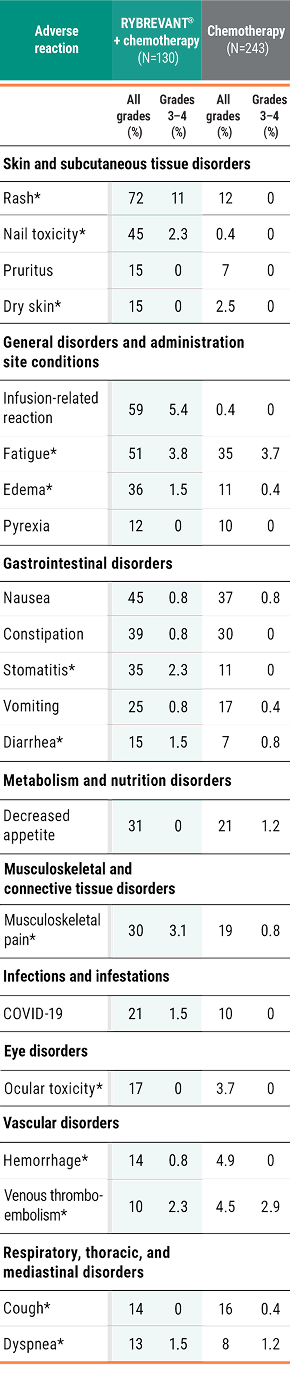
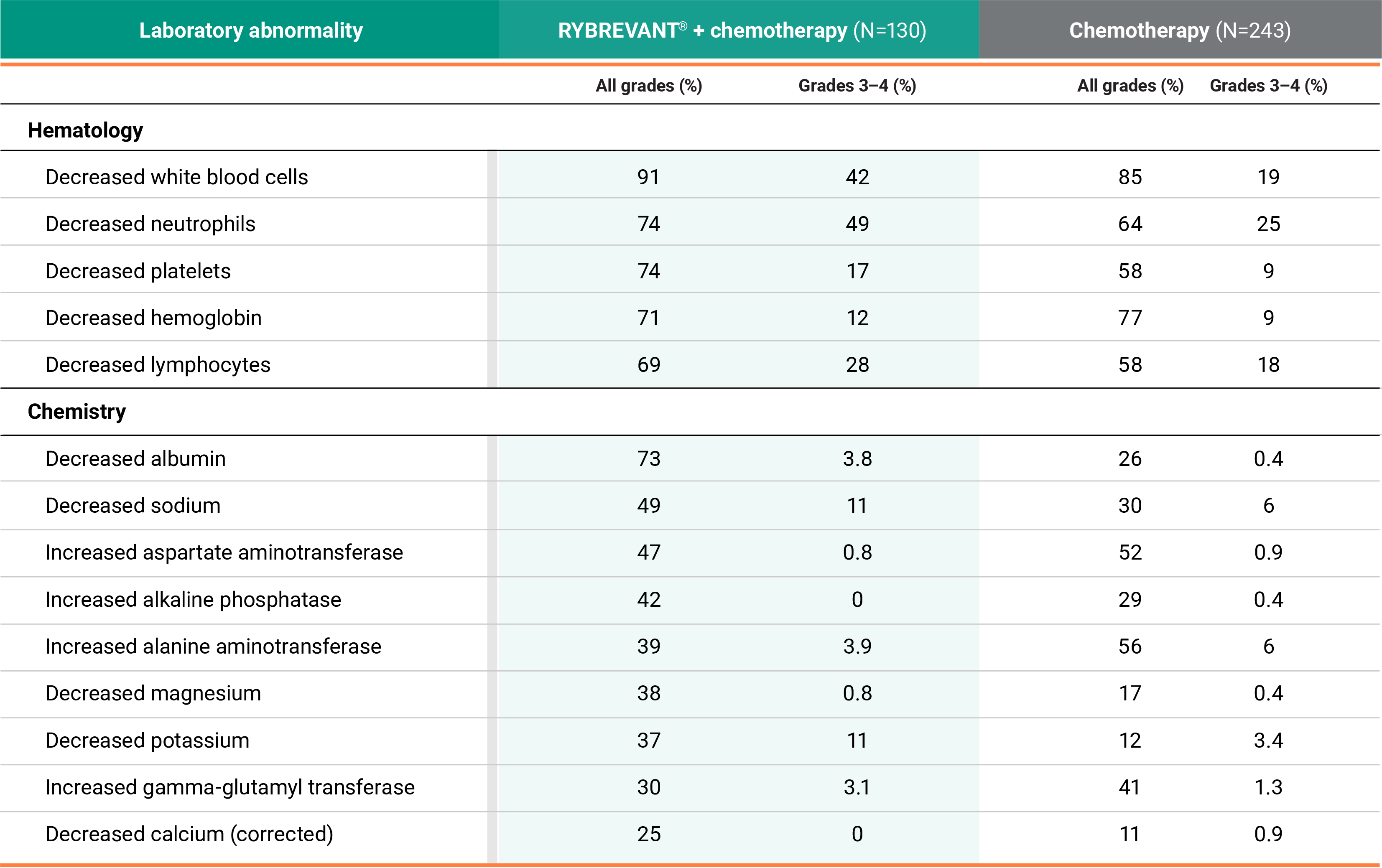
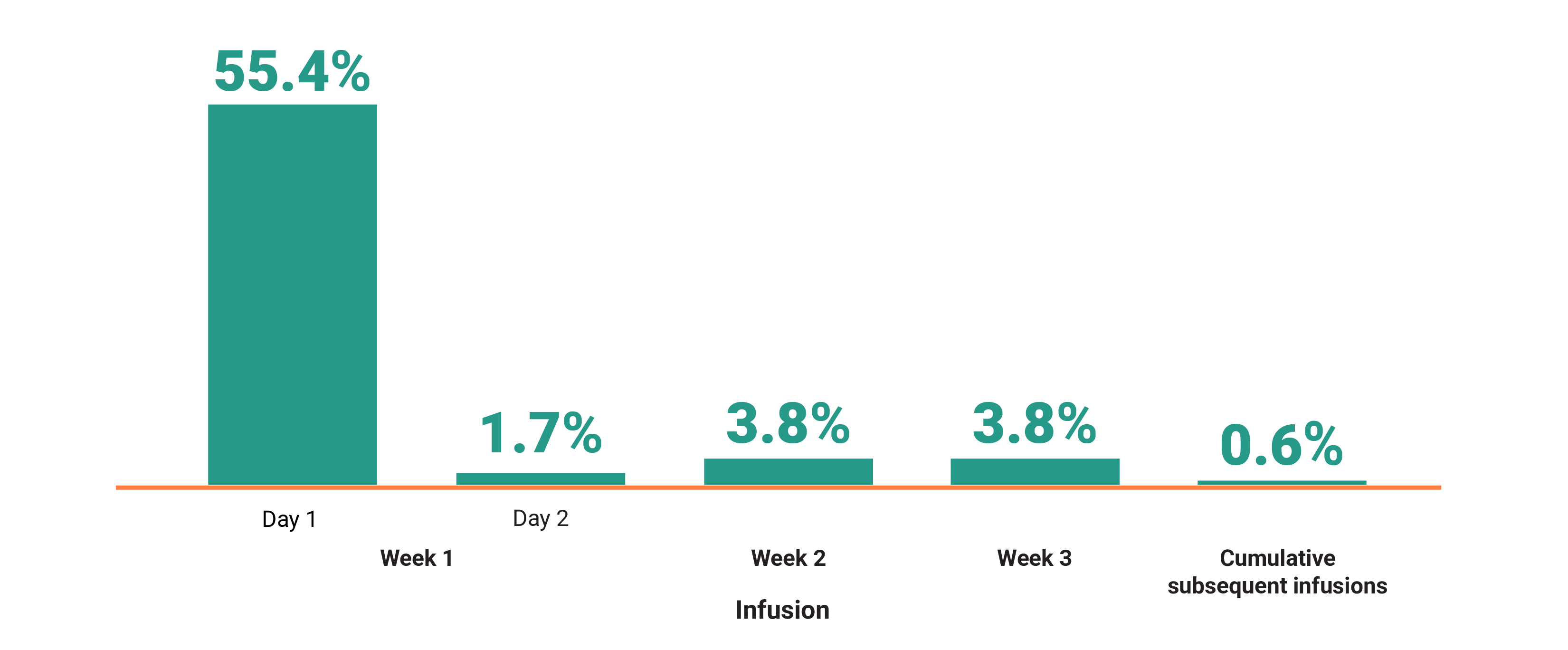
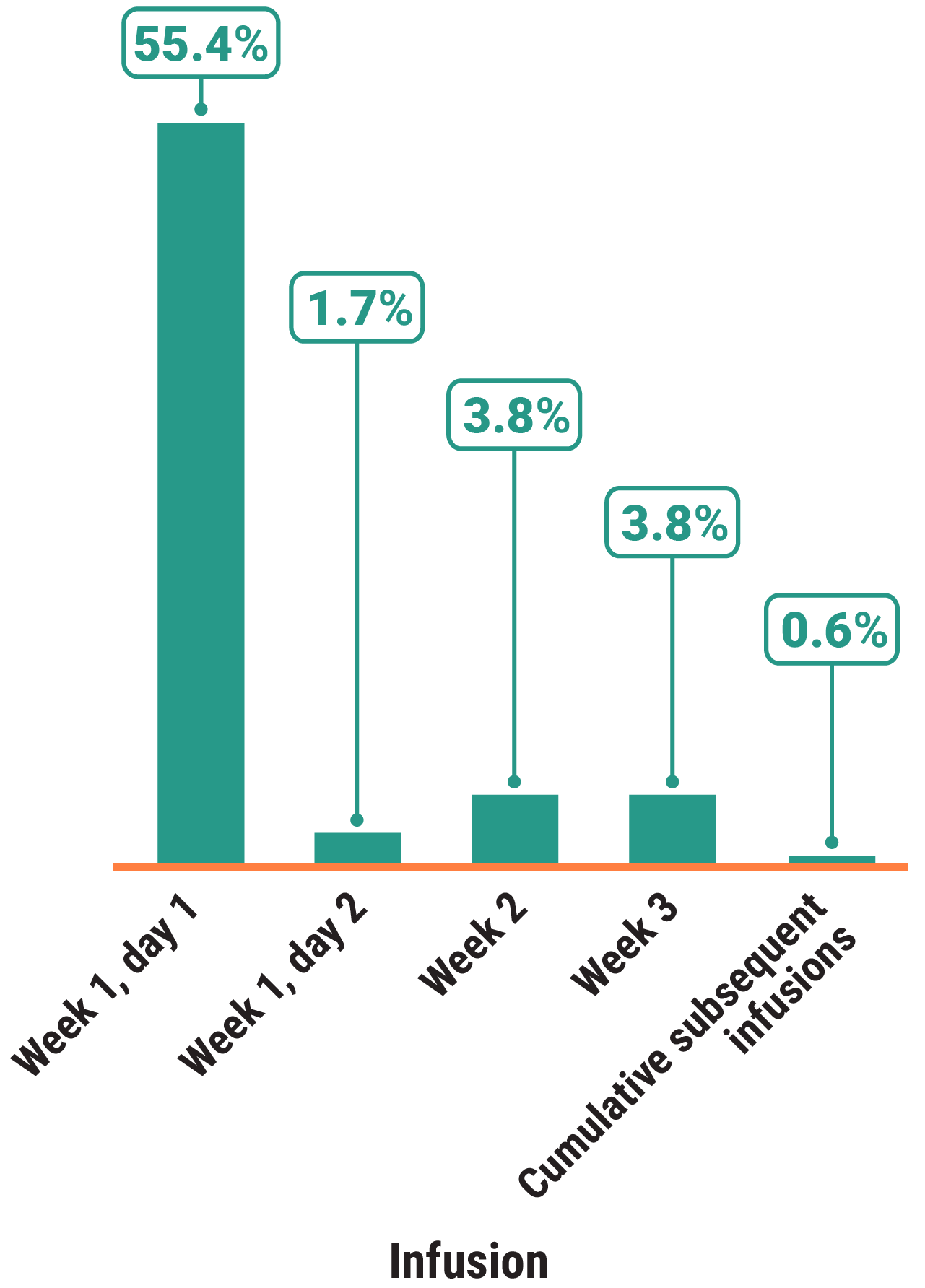
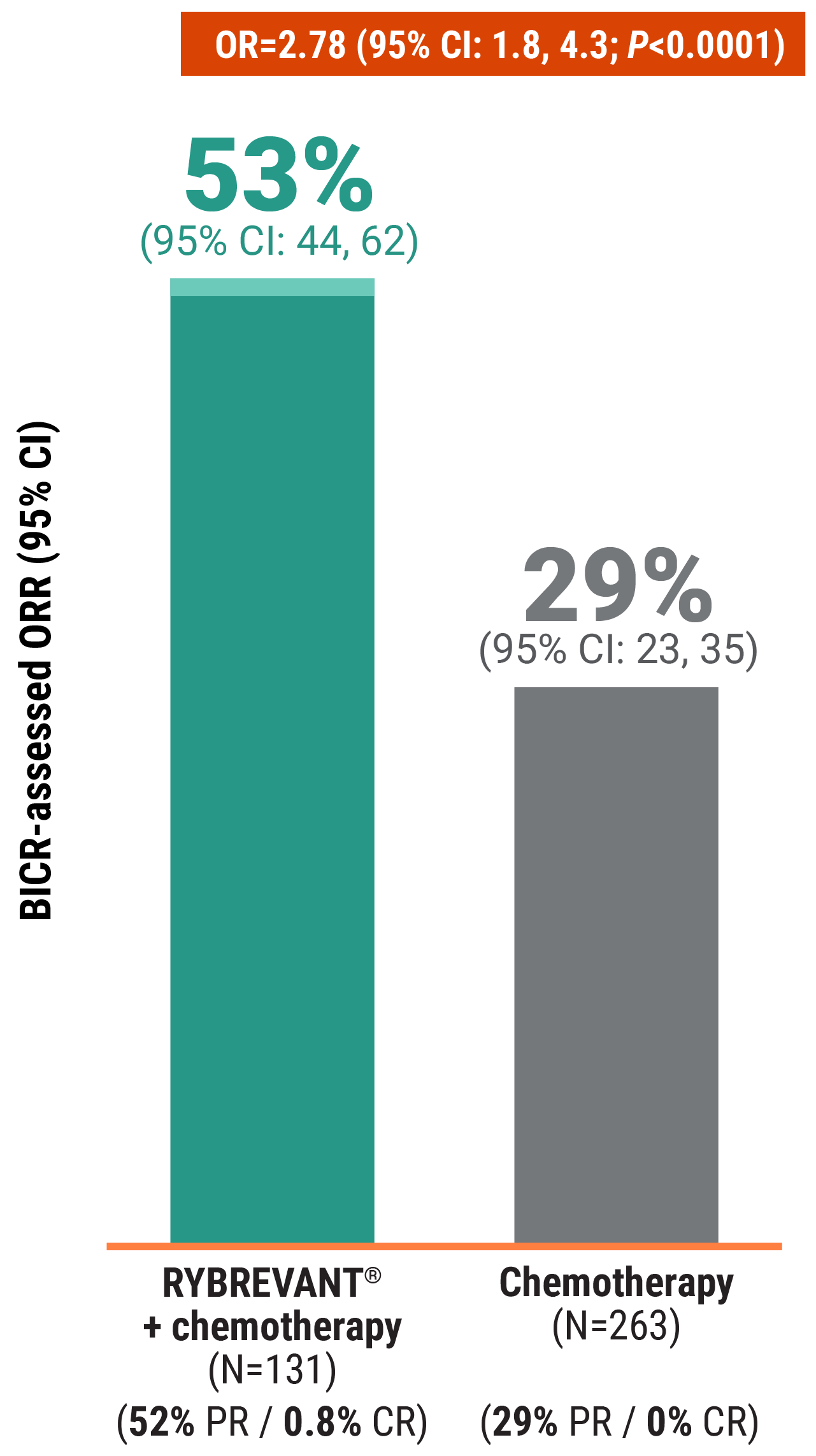Primary Endpoint
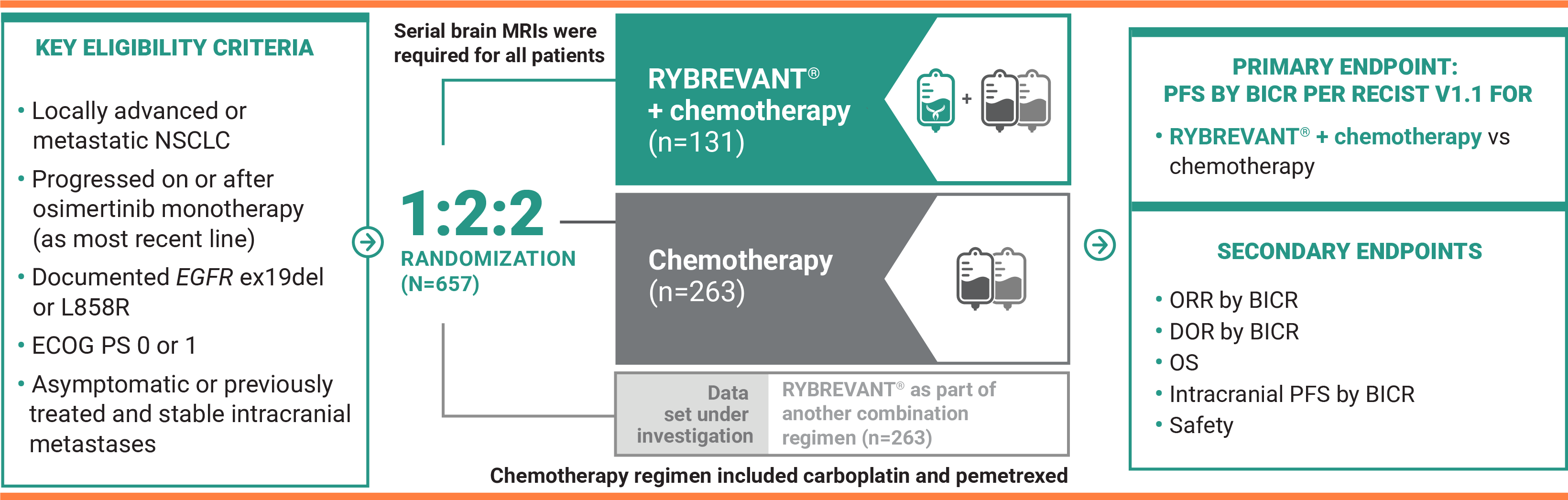
For previously treated adult patients with locally advanced or metastatic EGFR+ NSCLC
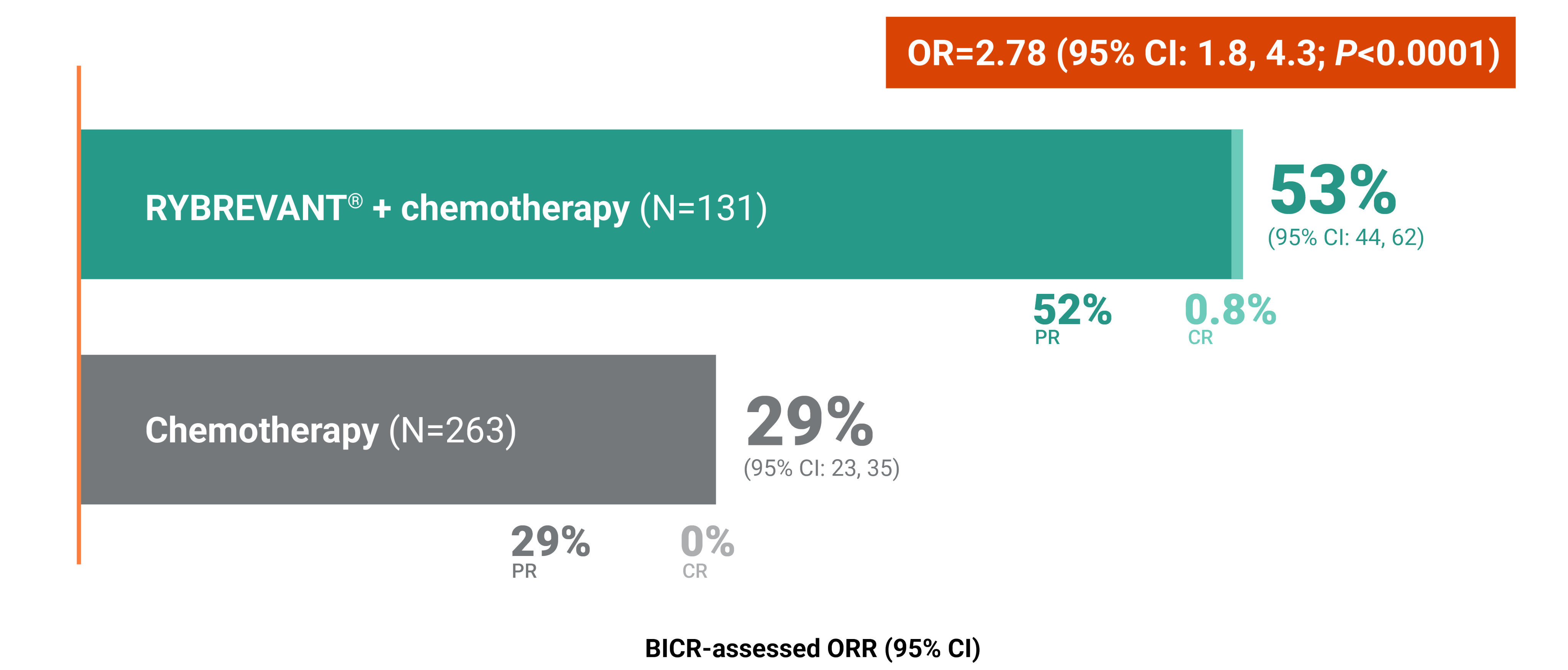
RYBREVANT® + chemotherapy—the first and only targeted combination to significantly improve PFS post-osimertinib1
RYBREVANT® + chemotherapy (carboplatin/pemetrexed) reduced the risk of progression or death by 52%1,2
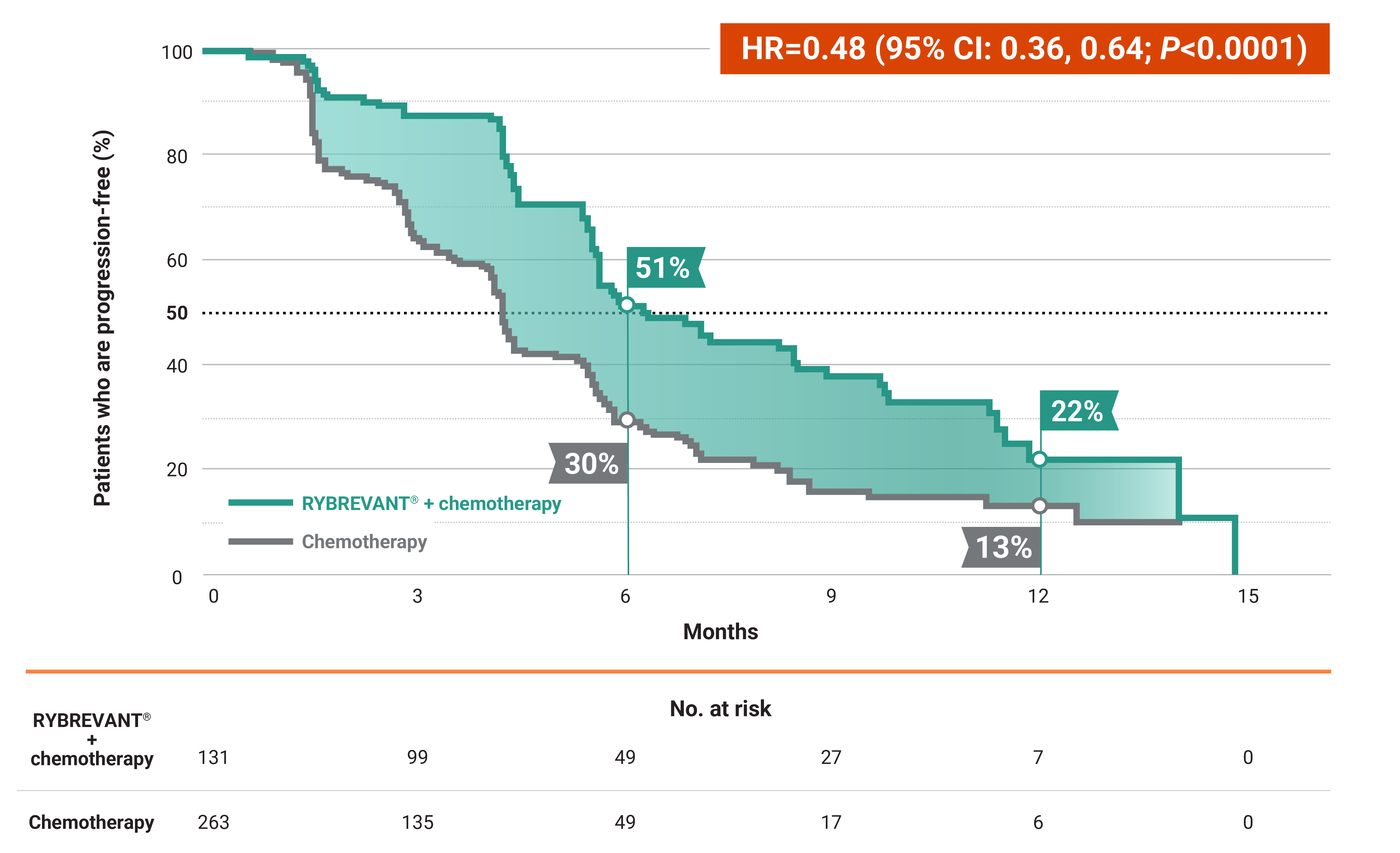
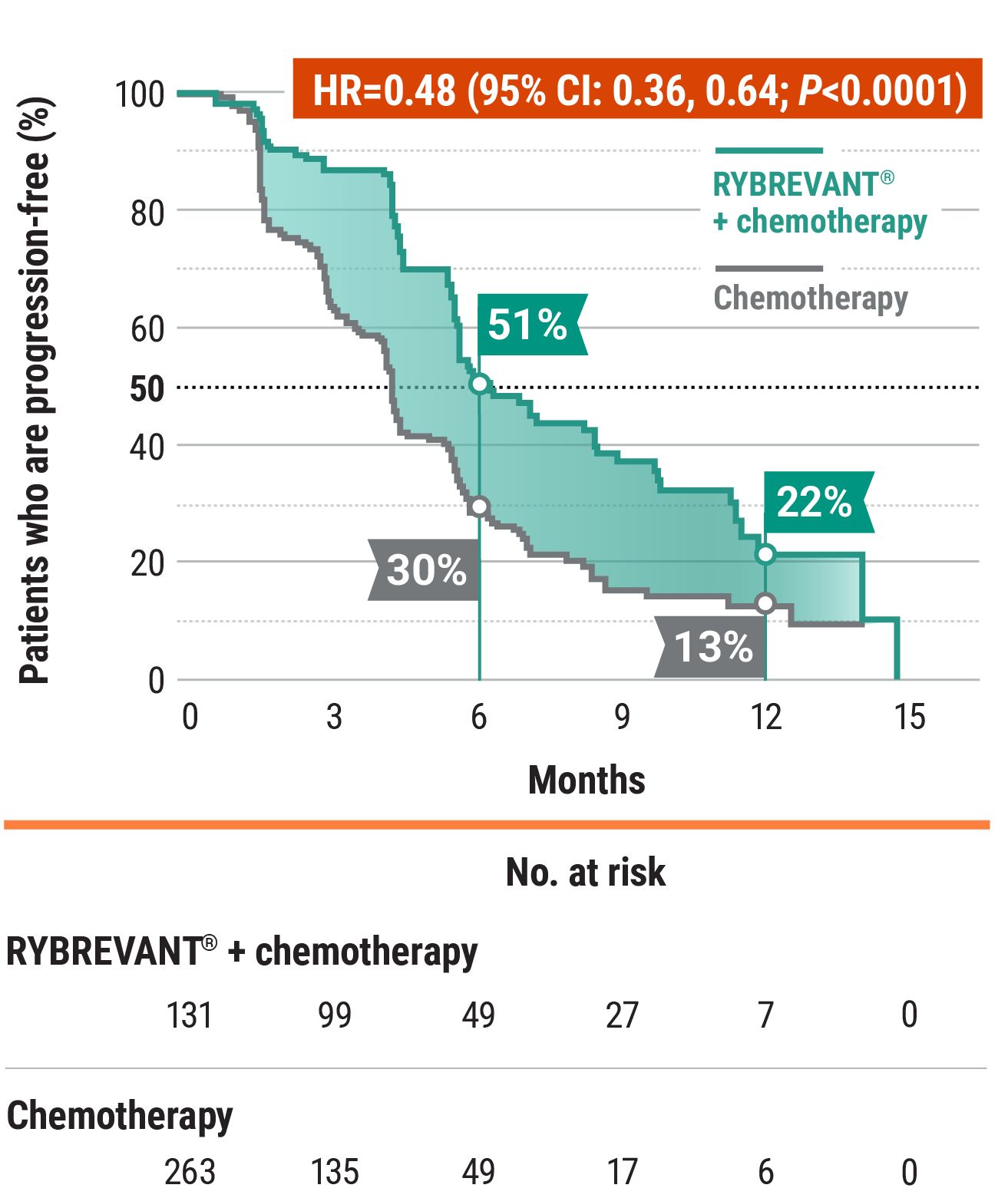
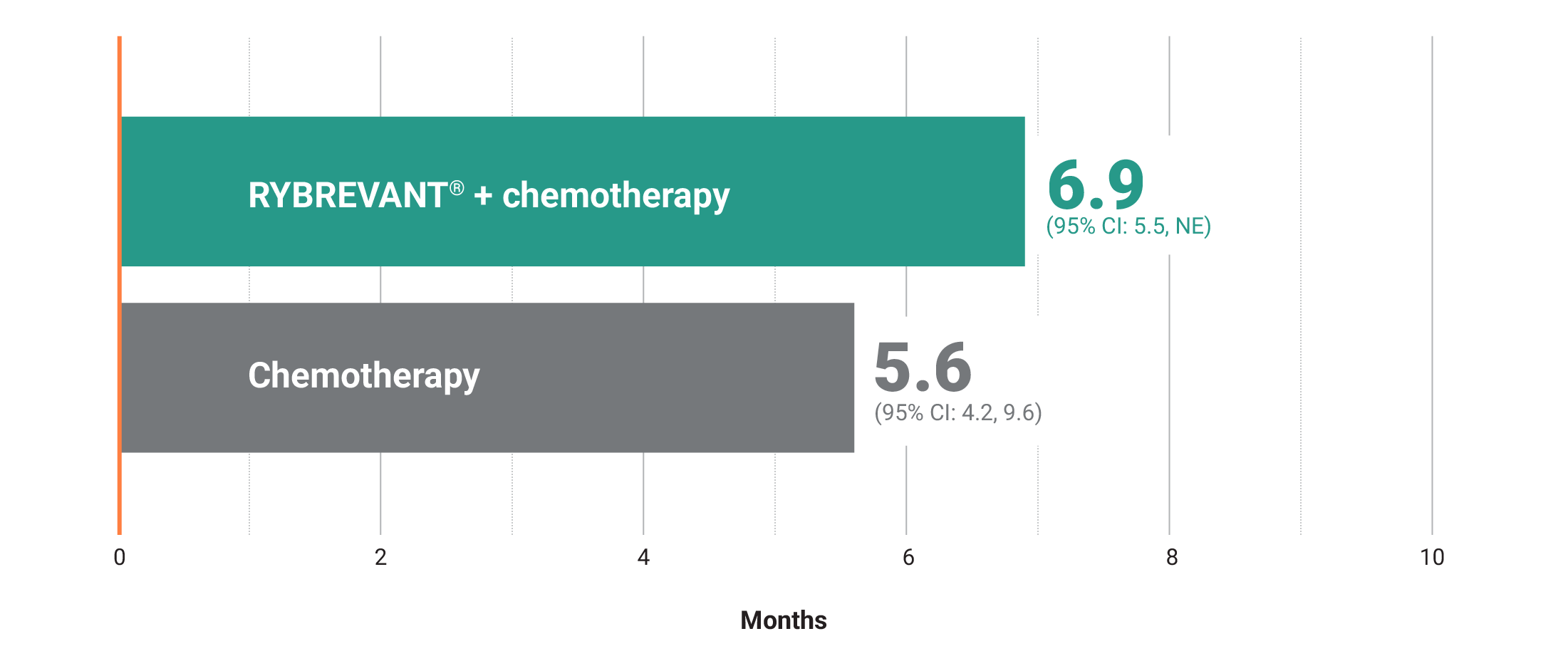
RYBREVANT® + chemotherapy demonstrated a median PFS of 6.3 months (95% CI: 5.6, 8.4) vs 4.2 months (95% CI: 4, 4.4) for chemotherapy alone2
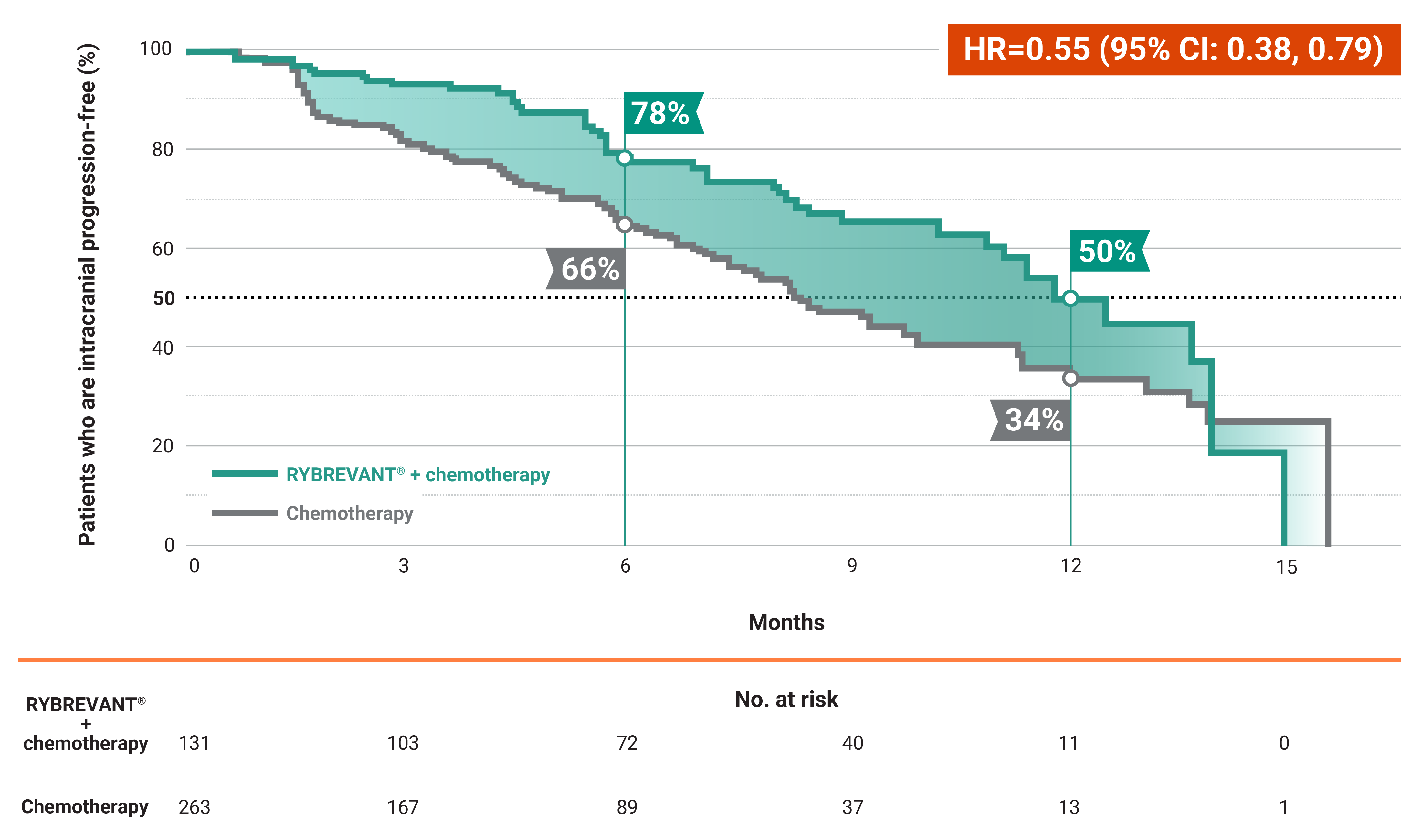
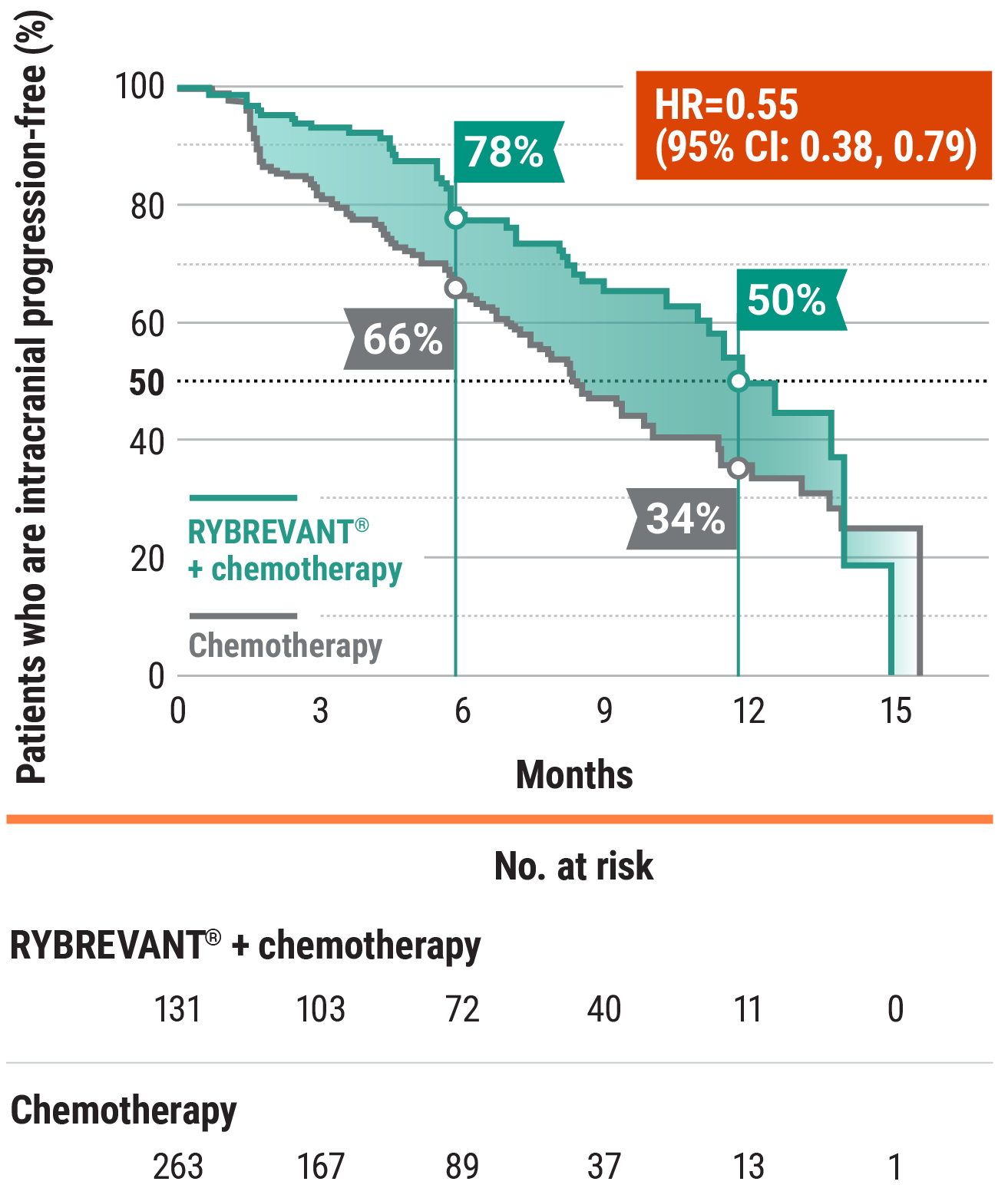
- At 6 months: 51% of patients were progression-free with RYBREVANT® + chemotherapy vs 30% of patients with chemotherapy alone2
- At 12 months: 22% of patients were progression-free with RYBREVANT® + chemotherapy vs 13% of patients with chemotherapy alone2
*Chemotherapy is carboplatin and pemetrexed.3
†EGFR exon 19 deletion or exon 21 L858R mutations.3
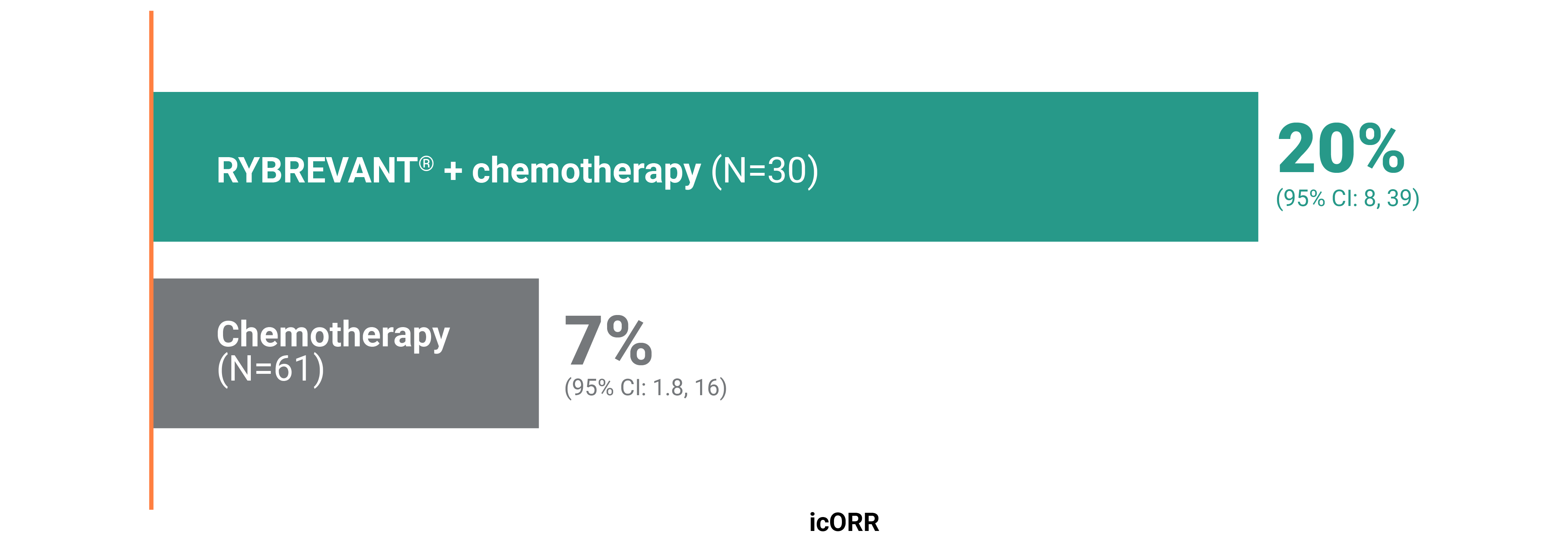
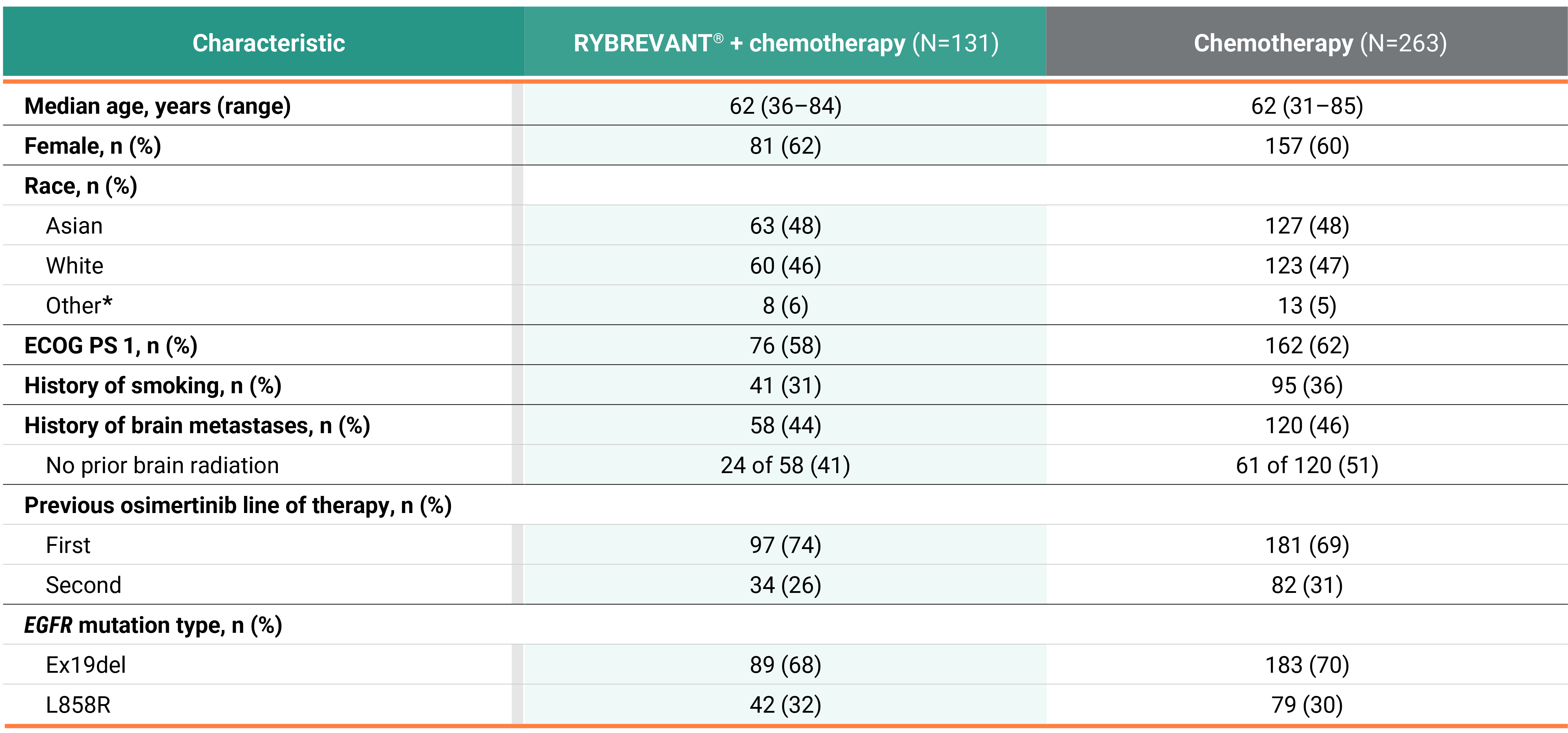
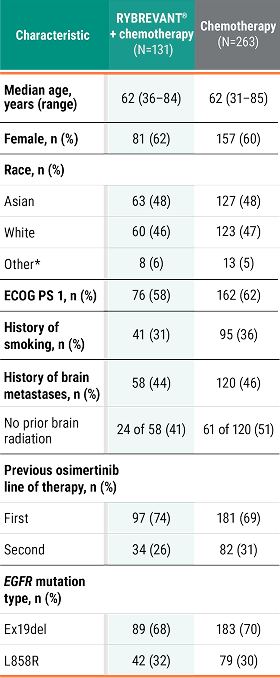
PFS across patient subgroups2


This was a prespecified analysis (except for EGFR mutation type) and was not powered to show statistical significance.
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ex19del, exon 19 deletion; HR, hazard ratio; mNSCLC, metastatic non–small cell lung cancer; NSCLC, non–small cell lung cancer; PFS, progression-free survival.